INTRODUCTION
Genetics is a field of high interest in dentistry, aimed at discovering the etiology of many diagnoses on a daily basis, as well as identifying therapeutic response patterns depending on genetic conditions. Thanks to advances in molecular biology, bone genetics and the physiology of connective tissue, orthodontic tooth movement is now better understood.1 Among the multiple alterations that can occur in the genome, the polymorphism of IL-1β has been identified as a genetic marker of periodontal disease,(2) as well as a modulator of bone resorption processes.3 In the field of orthodontics, the presence of IL-1β polymorphism has been associated with tooth movement speed,4 and a relationship with the predisposition to root resorption has also been found.5
Regarding root resorption, it is important to know the genetic risk factors that could make a patient a host susceptible to developing this pathology. One of the limitations patients usually refer to accept and start therapy has to do with treatment length. In addition, the evidence shows that there are natural risks in treatments that are too lengthy.6-8 To improve these situations, some techniques have been developed to accelerate orthodontic movement, including low intensity laser irradiation, mechanical vibration devices, drug therapy and even selective alveolar decortication techniques to produce controlled bone injury.9
The objective of this systematic review is to evaluate the effect of the IL-1β polymorphism on movement speed and/or root resorption in patients treated with orthodontics.
METHODS
Search strategy
A specific search was conducted to identify any relevant studies based on a combination of keywords and Boolean terms associated with the Cochrane Highly Sensitive Search Strategy (CHSSS) to identify randomized trials and studies in the Cochrane Central Register of Controlled Trials, Medline, Scopus and Embase. The following MESH terms were used: “orthodontic” AND “interleukin-1beta” AND “root resorption” OR “speed”, applying filters in the “humans” species as a result of the PICO criteria for the research question.
The list of references of all retrieved fulltext documents were explored in search of relevant documents that could have been lost during the electronic search. The titles and abstracts were examined prior to the revision of full texts. A positive exclusion method was used for the inclusion of articles, excluding only those publications that did not meet one or more inclusion criteria. Gray literature was not reviewed.
Inclusion criteria
Studies in men and women with orthodontic treatment, evaluating the effect of the presence of IL-1β polymorphism (interleukin 1 beta [Homo sapiens (human)] +3954) on movement speed and/or root resorption. Clinical studies, case-control studies, cross-sectional studies and cohort studies published in scientific journals in the English language to date were included.
Exclusion criteria
The following articles were excluded: animal studies, in vitro studies, studies on any metabolic product different to IL-1β, studies not evaluating root resorption or speed movement during orthodontic treatment. Review articles, systematic reviews or metaanalyzes were not considered.
Studies were selected according to the criteria specified above. Duplicate publications were identified to eliminate bias, comparing authors’ names, place and scope, specific details of the interventions, number of patients, baseline data, and date and duration of the study. All selected articles were included in the Mendeley® bibliographic reference manager, removing duplicate records from a same report. Titles and abstracts were then examined to eliminate clearly irrelevant reports. The full text of selected reports was retrieved to verify the studies’ degree of compliance with the eligibility criteria. A final decision was made, and data were obtained from the selected studies. The final decision on the studies to be included was taken independently by the two reviewers, with only one of them being an expert in the field to reduce evaluation bias concerning the relevance and validity of articles. Disagreements were solved based on the eligibility criteria; failure to meet a single criterion was enough for a study to be excluded from the review. Search records and selected articles were verified according to the PRISMA declaration.10
Data extraction
Titles and abstracts of all selected studies were evaluated during the initial search, excluding those not meeting the inclusion criteria and duplicates. A list of pre-selected articles was obtained. The inclusion and exclusion criteria were reassessed based on the full text of these shortlisted articles. Once the selection of final articles was completed, a data extraction table was created in a Microsoft Excel® database, recording the following variables: authors’ names, year, journal, country, and study design. A qualitative analysis was performed on the results obtained in the database.
RESULTS
Description of included studies
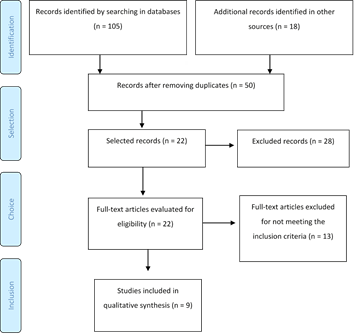
The literature search and selection process are shown in the PRISMA flowchart (Figure 1). The searches conducted in the Cochrane Central Register of Controlled Trials, Medline, Scopus, and Embase databases and the manual search yielded a total of 123 articles. After removing duplicates, 50 articles remained. The titles and abstracts were reviewed, excluding 28 articles that did not meet all the inclusion criteria: 2 articles made in biomodels, 2 that did not link the presence of IL-1β to movement speed or root resorption, 10 articles evaluating other metabolic products different to IL-1β and 14 among systematic reviews, technical descriptions, and summaries of published events. Full texts of 22 selected articles were retrieved to determine eligibility.
The full texts showed that 13 additional articles failed to meet all the previously established inclusion criteria, and 9 articles were finally included: six retrospective observational studies and three prospective studies (Table 1). These were published between 2001 and 2016. All the studies were conducted in universities: 5 in the United States, 2 in Latin America (Brazil and Colombia), 1 in Spain and 1 in Japan.
Table 2 lists the characteristics of studies in relation to orthodontic tooth movement, namely: type of orthodontic treatment, movement speed, characteristics of participants, treatment or follow-up time, sample characteristics to measure IL-1β, results, relationship with movement speed and IL-1β levels. The studies compare two groups corresponding to cases and controls, which were studied during the same period.
Table 3 lists the characteristics of studies in relation to root resorption: the stage of orthodontic treatment in which the root resorption measurements were made, characteristics of participants, characteristics of the sample to measure IL-1β, results of IL- 1β levels with root resorption.
Table 1
Original articles on IL-1β polymorphism, orthodontic tooth movement and ERR
Table 2
Evaluation of IL-1β and orthodontic tooth movements: cases and controls
| Author | Participants (sex, age) | Orthodontic treatment | Movement speed | Time | Sample features | Genetic Results |
|---|---|---|---|---|---|---|
| Iwasaki LR, 200150 | n = 7 | Extraction of first upper premolar and continuous mechanical forces for the retraction of maxillary canines | Canine retraction at average speeds of 1.27 and 0.87 mm per month for 13 and 4 kPa stress, respectively. | Days 0, 1 and 3, followed by intervals of 14 days up to day 84. | Mesial and distal GCF of each experimental maxillary canine and one untreated individual (control). | Experimental Activity Index (AI) (IL-1β: IL- 1RA). IL-1β 634 ng/g of protein, IL-1RA was 173 µg/g of protein. |
| Iwasaki LR, 200541 | n = 10 (3 male, 7 female, 10 years 5 months to 30 years 11 months. | Continuous maxillary canine retraction forces of 13 kPa and 4, 26 or 52 kPa bilaterally | Movement speed: with 52 kPa was 0.065 mm/day and with 13 and 26 and 0.035 mm/day. kPa, 0.038 | Days 0, 1 and 3, and then intervals of 14 days up to day 84. | Mesial and distal GCF of each experimental maxillary canine, and of one untreated individual (control). | The proportion of IL-1β and IL-1RA in GCF accounted for 56% of the variance in vt in growing subjects and 72% in adult subjects. |
| Iwasaki LR, 20064 | n = 10 (5 male, 5 female, 15 years old (±3 years, 8 months) | Bilateral removal of first upper premolars and distal movement of maxillary canines, with passive anchorage devices attached to the upper posterior teeth, and a custom vertical loop. | Canine retraction with average speeds of 4.14±0.19, 6.36±1.32 and 5.66±1.38 mm distal movement on day 84 for 13, 26 and 52 kPa respectively | Days 0, 1 and 3, and then intervals of 14 days up to day 84. | Mesial and distal GCF of each experimental maxillary canine, and one untreated individual (control). | For growing subjects, SWB IL1RA was correlated with v t (R=0.70- 0.72), and for AI SWB and IL-1B the concentrations were correlated with AI GCF (R=0.73- 0.78). |
| Iwasaki LR, 200961 | n = 33 (21 female and 12 male) 14.8 ± 3.9 years | Extraction of first maxillary premolars and distal movement of maxillary canines. | Distal compressive stresses of 4, 13, 26, 52 or 78 kPa on maxillary canines, and 0.063 mm/day as the maximum mean associated with tooth movement speed. | Days 0, 1 and 3, and then intervals from 14 days up to day 84. | GCF of two experimental sites, distal from each maxillary canine, and a control site, interproximal from a mandibular canine or an adjacent tooth. | Three important factors affected the speed at the 15% level: IL-1β genotype (+3954) (p = 0.039), AI (p = 0.0005) and IL-1RA. |
| Yamaguchi M, 200662 | n = 9 (3 male, X = 21.3 ± 2.8 years; 6 female, X = 23.1 ± 2.4 years) | Removal of first upper premolars before placing supports and wires. Initial force of 250 g. The canine subjected to distal movement was used as experimental tooth and the contralateral canine served as control. | Tooth movement speed was 1.5 ± 0.4 mm for 168 hours (7 days). | First 168 hours (7 days) of treatment. | Strips of paper inserted at 1 mm in the gingival sulcus for 1 minute. | The mean values of SP and IL-1β for treated teeth were significantly higher after 8, 24 and 72 hours. |
Table 3
Evaluation of IL-1β and root resorption: cases and controls
| Author | Orthodontic treatment | Root Resorption | Participants (age, gender) | Sample Characteristics | Results |
|---|---|---|---|---|---|
| Al-Qawasmi, 20035 | Pretreatment and post-treatment time 2.82 years (SD±1.09) | Group of cases >2 mm and unaffected subjects <2 mm. | n = 118 (X = 12.1 years (SD ± 1.89), male 36, female 70) | Scaling with sterile nylon bristle brush on tooth with maximum ERR. | Group affected by ERR occurred on the IL-1β (1.1) genotype (72%), followed by the (1.2) genotype (39%); the lowest percentage (0%) was on the (2.2) genotype. |
| Bastos Lages EM, 200955 | During comprehensive orthodontic treatment (straight arch technique) | Group of cases (n = 23) with at least 1 maxillary incisor with ERR of >2mm, and Control group (n = 38), with ERR <2 mm in the central and lateral maxillary incisors. | n = 61 (X = 18.9 years (SD±5.2) | Scaling with sterile wooden spatula on oral mucosa. | Polymorphism of the IL-1β gene is associated with root resorption. |
| Iglesias-Linares A, 201256 | Root canal treatment followed by full orthodontic treatment (straight arch technique). Time 27.21 months (±4.9 months) | Group of cases (n = 39) ERR presence >2 mm, Control group (n = 54) absence of ERR >2 mm after orthodontic treatment. | n = 93 (X = 24 years 1 month (5 years, 5 months) | Intraoral scaling using a sterile mouth swab. | In the control group, frequencies of the IL-1β genotype were less common than in the group with the presence of RRE. |
| Lince Vides F, 201659 | Orthodontic post-treatment. | Group of cases (n = 13) had post-treatment ERR and Control group (n = 22) did not develop ERR at the end of orthodontic treatment. | n = 35 (X = 28.1 years (SD±11.5), male 11, female 24) | Scaling with sterile swab on the oral mucosa. | There was no statistically significant difference between the presence of IL-1β gene polymorphism and ERR (p = 0.0926). |
DISCUSSION
Biophysical principles of orthodontic tooth movement
Many layers of interconnected reactions occur in and around the periodontal ligament and alveolar bone cells, changing the mechanical strength of molecular events (signal transduction) and orthodontic tooth movement. Osteoblasts and osteoclasts are sensitive communicators around the genome, capable of restoring the homeostasis of the system disturbed by orthodontic mechanics. Five micro-environments are altered by the orthodontic force: extracellular matrix, cell membrane, cytoskeleton, nuclear protein matrix, and genome.11
To understand the systems that involve orthodontic tooth movement, one should start from the messenger ribonucleic acid (mRNA). Current technology allows the individual detection of mRNA transcripts per cell.12 Knowing the gene expression during osteoblast differentiation provides information on the interaction of genes related to bone metabolism, as well as useful information on the discovery of genes that cause bone metabolism diseases. Simultaneous gene expression is studied in commercially available microarrays that allow complementary binding and analysis of experimental DNA samples with known DNA sequences.13,14
Because bone is a mineralized tissue, all changes in external bone shape occur along vascularized periosteal surfaces through unadjusted anabolic and catabolic modeling situations. Modeling changes the shape, size and/or position of bones in response to mechanical loading and/or lesion.1 The discoveries in molecular biology, bone genetics and connective tissue physiology have provided a somehow better understanding of the dynamics of orthodontic tooth movement.15 While many genes control the complex osteogenesis process, RUNX2 is a transcription factor (TF) closely associated with the osteoblast phenotype.16 Other bone-forming genes encode growth factors (GF), bone morphogenetic proteins (BMPs), transforming growth factor beta (TGF-β), and associated internal signaling molecules-GF.17 It is important to note that differentiation and proliferation of pre-osteoblasts are separate processes controlled by different genes.18
Osteocytes, the most numerous cells in bone, are star-shaped cells enclosed within a lacuno-canalicular bone network and have been shown to function as mechanosensory cells in bones.19,20 Osteocytes are involved in dental movement in response to orthodontic force.21 They are an essential source of RANKL among periodontal tissue component cells, which regulate osteoclast formation during spongy bone remodeling in orthodontic tooth movement.22,23
Osteoclasts are specialized multinucleated cells that develop from monocyte hematopoietic cells. Their unique properties include adhesion to the bone matrix and the secretion of lytic enzymes and acids that destroy mineral structures and proteins. At least 24 genes and 60 proteins are involved in the positive and negative regulation of the function of osteoclastogenesis and osteoclasts.24,25
Roberts et al consider bone resorption on the surface of the periodontal ligament (PDL) a speed limitation in orthodontic dental movement. Clinical success also depends on normal osteoclast and osteoblast genes that correctly express necessary proteins in adequate amounts at appropriate times and places.15 The earliest marker of bone resorption could be IL-1β. A mutant gene of IL-1β could be associated with the low regulation of this important cytokine.26
Interrelation of genetic factors and orthodontics
Additional knowledge on the genetic and environmental factors that affect patient’s biology can allow better predictability and control of the direction, nature, and speed of dental movement in orthodontics. Genetic related research and technology allow a better understanding of tooth movement and associated phenomena, such as bone modeling and remodeling.
Patients requiring orthodontics due to malocclusion or dental esthetics may have a high prevalence of alveolar bone defects such as dehiscence, especially on the surface of canines and mandibular incisors. Their presence increases the risk of mucogingival defects.27 It has been found that orthodontic patients have a higher prevalence of gingival recession in the vestibular area of lower anterior teeth compared to untreated patients.28 Therefore, identifying susceptible sites and understanding the predisposing clinical scenarios before orthodontic therapy become important steps to support long-term periodontal health.29,30
Variable genetic characteristics include dental and periodontal anatomy, systemic health, and the physiological reaction to different stimuli. Recent advances in genomic technologies provide interesting research possibilities to reveal the genetic basis of differences in orthodontic tooth movement among humans.
The mechanical activation of bone cells is linked to many genes, which produce various enzymes, such as glutamate/aspartate transporter, inducible nitric oxide synthase, and prostaglandin G/H synthetase.31 The mechanically induced regulation of osteoblasts and cementoblast genes has been studied in a mouse model. The researchers demonstrated a definite temporal pattern of specific gene regulation of periodontal osteoblasts, mechanically stimulated to differentiate and deposit bone matrix. According to the researchers, the primary responses to mechanical loading are the induction of osteogenic differentiation and the increase in cell function, instead of an increase in the number of cells. These differential genetic responses provide functional markers for a distinction between the phenotypes of cementoblasts and osteoblasts.32
Specifically, a polymorphism in exon 5 (+3954) of the IL-1β gene results in 2 alleles that modulate the secretion of IL-1β.33,34 Being homozygous for allele 2 (A2, A2) and heterozygous (A1, A2) in IL1β (+3954), it produces 4 times and 2 times increases in IL-1β secretion, respectively, compared to being homozygous for allele 1 (A1, A1).33 The specific impact of the polymorphism on the secretion of IL-1RA is similar.
A polymorphism in the second region of the intron of the IL-1RN gene results in a variable number of tandem repeats of an 86-base pair sequence (VNTR 86pb) and therefore in different alleles. Having at least 1 copy of allele 2 (A2 +), it is associated with an increase in the secretion of the IL-1RA protein. Based on evidence that polymorphisms in the gene are associated with IL-1β and the secretion of IL-1RA, and that the amounts of IL-1β and IL-1RA in GCF are associated with tooth translation speed (vt), the interindividual variability in tooth movement speed is likely to be regulated by genetic background.35,36
Variables affecting orthodontic tooth movement
The variables associated with tooth movement speed and patients’ individual characteristics have been identified, but have not been systematically and rigorously investigated to date. Regarding clinical evidence, it has been found that tooth movement is slower in adults than in younger individuals undergoing orthodontic treatment.37,38 Variability in tooth movement rate among individuals has been observed using the same force in studies in human beings.39-41 These results strongly suggest that individual specific characteristics are important for the biological responses that result in bone remodeling when orthodontic forces are applied.
In general, conventional orthodontic treatment ranges from 18 to 30 months in length.42,43 Tooth movement speed depends on the biological response of tissues to the mechanical stress produced by orthodontic forces on periodontal ligament and alveolar bone.44 This biological response is the product of molecular events that involve the synthesis and local release of several biomarkers and cytokines.37,45
Cytokines are extracellular signaling proteins that act on nearby target cells in low concentrations in an autocrine or paracrine form in cell-to-cell communications. Cytokines that are found to affect bone metabolism, and thereby orthodontic tooth movement, include interleukin 1 (IL-1), interleukin 2 (IL-2) interleukin 3 (IL-3), interleukin 6 (IL-6), interleukin 8 (IL-8), tumor necrosis factor alpha (TNFα), gamma interferon (IFNγ), and osteoclast differentiation factor (RANKL). The most potent among them is IL-1, which directly stimulates the function of osteoclasts through IL-1 receptors, expressed by osteoclasts. The secretion of IL-1 is activated by various stimuli, including neurotransmitters, bacterial products, other cytokines, and mechanical forces.37
IL-1 is a polypeptide produced primarily by mononuclear phagocytic lineage cells, and it usually promotes pro-inflammatory responses.46 IL-1 comes in alpha and beta forms. Of these two forms, IL-1β is believed to be more potent for bone resorption and bone formation inhibition,47 and, consequently, its role in orthodontic tooth movement has been the focus of previous studies.48,49
The ratio of 2 inflammatory IL-1β mediators and IL-1 receptor antagonist (IL-1β / IL-1RA), as well as polymorphisms of specific IL-1 gene groups have been highlighted as important determinants for movement speed of the tooth. IL-1β is a potent stimulator of bone resorption in vitro and in vivo, inducing osteoblasts to promote osteoclast activity.41 Iwasaki4,41,50,61 reported that cytokine concentrations in gingival crevicular fluid at dental movement sites compared to control group showed maximum values of IL-1β within 3 days of the application of force, suggesting that tooth movement speed is related to the concentrations of IL-1β and its balance with IL-1 RA.
External root resorption (ERR) related to IL-1β polymorphism
ERR is a pathological process with a multifactorial origin that is related to the permanent loss of root dental structure in response to mechanical, inflammatory, autoimmune or infectious stimuli.51 Only until 1997 Harris et al52 found in a group of 103 orthodontic patients that the polymorphism of IL-1β is associated with the pathogenesis of ERR. A potential for the diagnosis of orthodontic patients who may have a predisposition to this pathology was found in genetic studies.
In orthodontic treatment, ERR can occur mainly in the maxillary incisors, due to periodontal ligament compression. This compression causes a decrease or interruption of microcirculation, causing sterile necrosis. During the extraction of this necrotic tissue by macrophages and clastic cells, the integrity of roots can be damaged.53 Molecular studies have helped us understand these mechanisms; however, the risk factor directly associated with ERR during orthodontic treatment has not yet been identified. Only recently was it shown that polymorphism of IL-1 genes is associated with an increased risk of ERR during orthodontic treatment.54
The evidence of association of IL-1β polymorphism with ERR according to patient’s predisposition helps establish preventive measures during orthodontic treatment, such as the magnitude and duration of the force to be applied. IL-1 has been frequently associated with inflammatory events in connective and bone tissues.15 In addition, IL-1β has been characterized as a potent bonebased cytokine and is implicated as a key component of the complex pathways that lead to ERR.53 Al-Qawasmi et al5 and Bastos et al55 agree that the relationship of molecular mechanisms with this clinical pathology suggests that IL-1β stimulates clastic cells during orthodontic movement and that low production of IL-1β could result in less bone resorption.
Confirming the presence of genetic polymorphisms in patients before starting orthodontic treatment could be an interesting strategy to prevent ERR. Iglesias-Linares56 found a lower presence of IL-1β in patients who did not present ERR than in the group that did present the pathology. These findings are similar to those reported by Bastos-Lages et al,55 who found an association between polymorphism of the IL-1β gene and ERR in a Brazilian population, suggesting that the allele 1 has a greater predisposition in subjects with ERR. In this sense, the polymorphism analyzed in this study has been reported as a functional polymorphism, that is, it can alter the function of the IL-1β gene among the models that have been proposed to explain the mechanism by which the IL-genotype 1β modulates the degree of ERR experienced during orthodontic dental movement. It has been suggested that this polymorphism affects the production of IL-1β in the case of the C allele, resulting in a relatively less catabolic bone modeling at the cortical bone interface of the periodontal ligament because of the decrease in the number of osteoclasts associated with lower levels of this cytokine.55
However, it is not yet completely clear whether the polymorphism of the IL-1B gene is related to the presence of ERR in individuals undergoing orthodontic treatment; therefore, further examinations including a greater number of variables are needed. The contradictory reports found in the literature could be explained because polymorphisms can substantially change depending on specific race or populations; in this sense, it has been suggested that Asian populations have a higher frequency of the CC genotype of the IL-1β polymorphism than other ethnic groups. Similarly, Al-Qawasmi et al5 found that individuals with the CC genotype of the IL-1β polymorphism in the Caucasian population have a high risk of ERR.
On the other hand, Tomoyasu et al57 found no statistically significant association between IL-1β polymorphism and the presence of ERR in the Japanese population. Linhartova et al58 also failed to establish an association between IL-1β polymorphism and ERR in orthodontic patients.
Methodological differences and study population can decisively influence the results. The Al-Qawasmi et al5 study in 118 participants found statistically significant differences. On the other hand, Lince59 found no association in a small sample of 35 subjects. These varying results may be due to differences in treatment protocols, sampling, laboratory analysis and statistical analysis. Wu et al60 reported that the frequency distribution of genetic polymorphisms is significantly different among different ethnic groups; for example, the frequency of the TT genotype varied from zero in the Asian group to 32.8% in the South American group. Variation in genotype frequency among ethnic groups may contribute to the lack of a significant association in general.
CONCLUSIONS
According to the reviewed literature, it seems to exist an association between the polymorphism of the IL-1β gene with external root resorption and tooth movement speed.
It is necessary to conduct experimental studies in humans in order to further clarify the actual characteristics of this possible association, conducting randomized controlled clinical trials involving mechanical, biological and genetic factors that can explain the differences in bone remodeling and tooth movement among patients.