Introduction
Respiratory disorders are associated with risk of hospital admission (1), high economic burden, costs to the health system, and costs with drug acquisition (2,3), in addition, to be suggested as risk factors for the low quality of life in elderly people (4).
The world has been currently experiencing the new pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and COVID-19 (5). Studies have raised hypotheses that older people and the presence of comorbidities, such as other respiratory system disorders, high blood pressure, and diabetes mellitus, make the population more vulnerable to being affected by the COVID-19 infection (6). Furthermore, the presence of these risk factors can be associated with a more severe manifestation of the COVID-19 infection, which may require mechanical ventilation and admission to the intensive care unit, which finally may evolve to death (7-9).
Recent experience also suggests that the presence of uncontrolled comorbidities contributes to the poor prognosis of COVID-19 in the elderly. (10). Therefore, in the scope of patient safety, the most effective strategy is the monitoring and control of comorbidities considered to be risk factors to COVID-19 infection (7). Nevertheless, adequate management of comorbidities can be impaired in the elderly due to the aging process and the presence of multimorbidity (11) and polymedication (12), which may favor the prescription of potentially inappropriate medications (13), the occurrence of drug interactions (14), and adherence issues (15).
The prescription and use of potentially inappropriate medications among elderly people with respiratory disorders are frequent. It was found that about 55% of the elderly diagnosed with chronic obstructive pulmonary disease (COPD) or asthma were in use of inappropriate medications at hospital admission (16). Besides, a Brazilian follow-up study identified that seven in every ten elderly people diagnosed with COPD were in use of at least one inappropriate medication (17).
In this context, a recent review reported drug interactions and potentially inappropriate medication in older adults with mental and behavioral disorders (18); however, little is known regarding the elderly with respiratory disorders. Therefore, considering the burden associated with respiratory disorders (2,3), the high prevalence and mortality among elderly people with these disorders (5), the international concern with COVID-19 (5), the frequent use of potentially inappropriate medications, and others safety risks associated (16), a systematic scoping review was conducted to identify drug interactions, of potentially inappropriate medications, with respiratory disorders reported by explicit potentially inappropriate medications criteria-based tools.
Methods
A systematic scoping review was conducted following the recommendations of the Joanna Briggs Institute guidelines (21) and of Cochrane Collaboration (Cochrane Handbook for Systematic Reviews of Interventions) (20) which guide the processes for data extraction and synthesis. Furthermore, PRISMA-ScR (Preferred Reporting Items for Systematic reviews and Meta-Analyzes extension for Scoping Reviews) was considered for this reporting (22). The protocol of this systematic scoping review was previously outlined and published (19).
Search strategy and selection criteria
This systematic scoping review included studies that developed and/or validated explicit potentially inappropriate medications criteria-based tools for older adults and reported potentially inappropriate medications and/or drug interactions involving respiratory disorders. Moreover, potentially inappropriate medications with concerns and adverse drug reactions related to respiratory diseases, and therapeutic management were considered. There was no limitation on publication time, the country in which the tools were developed, level of health care or professional involved in potentially inappropriate medications and/or drug interaction definition or assessment.
The search strategy was carried out on PubMed and Scopus in February 2020. The search strategy, including all identified keywords and descriptors, was adapted for each database included and detailed in Table 1 of supplementary material from reference 23. Articles written in non-Roman characters (i.e., Russian, Japanese, Chinese) were excluded during selection process.
Clinical trials, observational studies, and studies conducted by a panel of experts who proposed explicit potentially inappropriate medications criteria-based tools were considered. Editorials, commentaries, letters, news, abstracts from conference proceedings, theses and dissertations, implicit criteria, old versions of the tool, and drugs no longer marketed on the world were excluded.
For manual search, the reference lists of included articles and previously identified reviews were searched.
Studies selection, extraction, and data presentation
The identified records were imported in EndNote version X 7.2.1 (Clarivate Analytics, PA, USA) and exported to spreadsheets to recording the exclusion reasons. Two authors (M.F. and G.S.) performed the study selection process independently. Disagreements among authors at each selection stage were resolved through consensus discussion or with a third author (R.C.L.).
The data were extracted by two independent reviewers (M.F. and G.S.), using Microsoft Office Excel spreadsheets or Microsoft Office Word (Microsoft, Redmond, WA, USA). The extracted data included specific details about (i) characteristics of studies; (ii) distribution of tools by year and country; and (iii) potentially inappropriate medications, drug interactions, concerns, and therapeutic management (e.g., deprescription, therapeutic equivalent) according to the tools. Potentially inappropriate medications were classified according to commercialization and essentiality by Rename (Brazil) and WHO (world).
The results are presented in a narrative form and tables to assist in the presentation of the data.
Following the recommendations of the Joanna Briggs Institute (21), the methodological quality assessment was not carried out because, conversely to systematic reviews aimed at assessing effects, systematic scoping reviews aim to group all available evidence on a specific subject, regardless of methodological quality.
Table 1
Description of explicit potentially inappropriate medications criteria-based tools and drug interactions between potentially inappropriate medications and asthma, chronic obstructive pulmonary disease, and respiratory failure.
| Explicit potentially inappropriate medications criteria-based tools | Country | Setting | Tool development technique | Number of drug interactions | Rationale for drug interactions | Proposal of management for drug interactions | Inappropriate medication with concerns for respiratory disorders | Proposal of therapeutic management for inappropriate medication |
| Updating Beers criteria, 2003 (55) | US | NR | LR; DP-1R; DIS | 6 | 6 | 0 | 0 | 0 |
| Thai criteria, 2008 (56) | THAI | NR | LR; DP-3R | 6 | 6 | 0 | 1 | 0 |
| Kim criteria b, 2010 (57) | KR | AMB | PIM-I; DP-2R | 4 | 4 | 4 | 0 | 0 |
| Mimica criteria, 2012 (58) | CRO | NR | PIM-I | 4 | 2 | 4 | 0 | 0 |
| Mann criteria, 2012 (59) | AT | NR | DP-2R; DIS | 0 | 0 | 0 | 3 | 2 |
| Fialová criteria, 2013 (60) | CZ | HOSP | DP-3R | 7 | 7 | 1 | 0 | 0 |
| Clyne criteria, 2013 (61) | IE | AMB | LR; PIM-I; CS | 1 | 1 | 1 | 0 | 0 |
| STOPP/START Spanish version, 2015 (62) | ES | NR | PIM-I | 1 | 1 | 0 | 0 | 0 |
| STOPP/START v2, 2015 (63) | IE | NR | PIM-I; DP-2R | 1 | 1 | 0 | 0 | 0 |
| GheOP3S tool, 2015 (64) | BE | AMB | LR; DP-2R | 2 | 0 | 2 | 0 | 0 |
| The EU(7)-PIM list, 2015 (65) | EUR | NR | LR; DP-2R | 0 | 0 | 0 | 3 | 3 |
| STOPP/START Japanese, 2016 (66) | JP | NR | LR; DIS | 0 | 0 | 0 | 5 | 5 |
| Poudel criteria, 2016 (67) | AUS | LTI | PIM-I; LR | 0 | 0 | 0 | 1 | 1 |
| Brazilian Consensus of Potentially Inappropriate Medicines for the Elderly (68) | BR | NR | PIM-I; DP-2R | 1 | 1 | 0 | 1 | 0 |
| Korean List, 2018 (69) | KR | NR | PIM-I; DP-4R | 1 | 0 | 0 | 0 | 0 |
| Lista IFAsPIAM, 2018 (70) | AR | NR | LR; DP-3R | 0 | 0 | 0 | 6 | 5 |
| STOPP/START Sri Lanka, 2019 (71) | SL | NR | PIM-I; DP | 1 | 0 | 0 | 0 | 0 |
| Gonzalez criteria, 2019 (72) | ES | NR | LR; DP-2R | 0 | 0 | 0 | 1 | 0 |
| Chang criteria, 2019 (73) | TW | NR | LR; DP-2R | 2 | 2 | 0 | 0 | 0 |
[i]Explicit potentially inappropriate medications criteria-based tools: EU: European union; GheOP3S: Ghent Older People’s Prescriptions community Pharmacy Screening; IFAsPIAM: Ingredientes Farmacéuticos Activos Potencialmente Inapropiados en Adultos Mayores (in English, Potentially Inappropriate Active Pharmaceutical Ingredients in Older Adults); PIM: potentially inappropriate medications; STOP/START: Screening Tool of Older Persons' Prescriptions/ Screening Tool to Alert to Right Treatment; US: United States; Title: ADE: adverse drugs event; Country: AR: Argentina; AT: Austria; AUS: Australia; BE: Belgium; BR: Brazil; CRO: Croatia; CZ: Czech republic; ES: Spain; EUR: Europe; IE: Ireland; JP: Japan; KR: Korea; SL: Sri Lanka; THAI: Thailand; TW: Taiwan; Population: ELD: Elderly in general; Setting: AMB: Ambulatorial; HOSP: Hospital; LTI: Long Term Institutions; NR: Not reported. Tool development technique: CS: Case-study; DIS: Discussion with the experts; DP: Delphi panel without number of rounds; DP-1R: Delphi panel 1-round; DP-2R: Delphi panel 2-rounds; DP-3R: Delphi panel 3-rounds; DP-4R: Delphi panel 4-rounds; LR:Literature review.
Results
This systematic scoping review identified 2,370 records after removing duplicates, and ten records were included after a manual search. 2,273 records were considered irrelevant during screening, and 53 were excluded in the full-text evaluation. Although 54 records were considered relevant, only 19 reported drug interactions from potentially inappropriate medications and respiratory disorders and potentially inappropriate medications with concerns for the respiratory system. Therefore, 19 explicit potentially inappropriate medications criteria-based tools were included and are described in Table 1. The supplementary data of this manuscript was published in a public repository and available in the reference 23.
The tools identified were published between 2003 and 2019; developed on the European (n=10), Asian (n=5), American (n=3), and Oceania (n=1) continents; and for the elderly in general, without specific morbidities or clinical conditions.
Nineteen drug interactions between potentially inappropriate medications and respiratory diseases were identified. Although the International Classification of Diseases (ICD-10) for respiratory disorders comprises 100 morbidities, the tools reported drug interactions involving three morbidities, namely: asthma, COPD, and respiratory failure (Table 2).
Table 2
Description of drug interaction of potentially inappropriate medication and three morbidities of respiratory system (asthma, chronic obstructive pulmonary disease, and respiratory failure), rationale, and therapeutic management proposal.
| Drug/Pharmacological class [n of tool] (reference) | Rationale, related risks, and/or adverse drug events | Therapeutic management proposed the by tools |
|---|---|---|
| Asthma | ||
| Benzodiazepines [2] (56,58) | Worsening of asthma and respiratory depression (56,58). | Benzodiazepines (short or intermediate half-life) (58). |
| Beta-blockers [6] (56-58,60,64,73) | Worsening of respiratory diseases; heart failure; mucus production (57,58,60,64,73); and bronchoconstriction due to B2 antagonistic effects (56). | Cardioselective beta-blockers; and other pharmacological class of antihypertensive drugs (57,58,64). |
| Mucolytics [1] (58) | Increasing mucus production (58). | Not reported |
| Chronic obstructive pulmonary disease (COPD) | ||
| Antiasthmatics [1] (69) | Not reported | Not reported |
| Antitussives [1] (60) | Increasing mucus production (60). | Not reported |
| Benzodiazepines [4] (56,58,60,71) | Worsening of respiratory depression (56,58,60,71). | Benzodiazepines (short or intermediate half-life) (58). |
| Benzodiazepines: chlorazepate, clodiazepoxide, diazepam, halazepam, quazepam [1] (55) | Induce or exacerbate respiratory depression; adverse drug reactions in the central nervous system (55). | Not reported |
| Benzodiazepines: chlorazepate, diazepam [1] (57) | Induce or exacerbate respiratory depression; adverse drug reactions in the central nervous system (57). | Non-pharmacological therapy (57). |
| Beta-blockers [7] (56-58,60,64,68,73) | Bronchospasm; worsening of respiratory depression; worsening of respiratory symptoms (57,58,60,64,68,73); bronchoconstriction due to B2 antagonistic effects (56). | Calcium channel blockers; cardioselective beta-blockers; another pharmacological class of antihypertensive drugs (57,58,64). |
| Beta-blockers: propranolol [1] (55) | Induce or exacerbate respiratory depression; adverse drug reactions in the central nervous system (55). | Not reported |
| Mucolytica [1] (60) | Increasing of mucus production (60). | Not reported |
| Systemic corticosteroids [1] (61) | Systemic adverse drug reactions (61). | Inhaled corticosteroids (61). |
| Respiratory insufficiency | ||
| Benzodiazepines [2] (62,63) | Worsening of respiratory depression (62,63). | Not reported |
| Biguanides: metformin [1] (60) | Toxicity; lactic acidosis (60). | Not reported |
The most frequent pharmacological classes involved in the drug interactions were beta-blockers (n=11) and benzodiazepines (n=8), and the drugs clorazepate (n=2) and diazepam (n=2), both of benzodiazepines pharmacological class. The possible rationale and the mechanism for the occurrence of the drug interactions were reported for 18 of the 19 drug interactions found, with increased mucus production leading to bronchoconstriction due to antagonistic effects on B2 receptors, worsening respiratory diseases, and respiratory depression being the most frequent.
Despite there is no therapeutic management proposal for the potentially inappropriate medications involved in 15 drug interactions, the tools reported some options for managing beta-blockers and benzodiazepines. Regarding beta-blockers, the tools recommended the replacement for cardioselective beta-blockers, calcium channel blockers, angiotensin-converting enzyme inhibitors, and angiotensin II type I receptor blockers or another class of antihypertensive drugs. For benzodiazepines, it is recommended the use of short or intermediate half-life benzodiazepines. The rationale and therapeutic management reported in the tools for the drug interactions, and potentially inappropriate medication are described in Table 2.
Eight tools reported 17 potentially inappropriate medications with concerns for the respiratory system. The drugs most frequently reported were phenobarbital (n=3), propranolol (n=2), and theophylline (n=2), all associated with respiratory depression, exacerbation of the respiratory disorder, and induction of asthmatic attacks. Clinical monitoring of possible adverse drug events from phenobarbital use is recommended and, when possible, the use of antiepileptics (lamotrigine, valproic acid, levetiracetam, and gabapentin) is preferable. Angiotensin-converting enzyme inhibitors, angiotensin II type I receptor blockers, selective beta-blockers, and diuretics should be considered instead of propranolol. Besides, carvedilol may be the best choice in heart failure complicated by COPD. No pharmacotherapeutic equivalent was reported for theophylline, however, cardiac function monitoring is strongly recommended due to this drug's narrow therapeutic range. When possible, monitoring this drug's use to identify possible adverse drug events and starting non-pharmacological treatment was recommended (Table 3 and Figure 1).
Twenty-two drugs and nine pharmacological classes were reported in drug interaction and/or associated with adverse drug events with respiratory disorders. Among them, nine and eight drugs and nine pharmacological classes are standard as essential in the world (WHO) and Brazil (Rename), respectively. Besides, the tools proposed 22 therapeutic equivalents for the potentially inappropriate medication identified, being 18 standards as essential by Rename and 16 by WHO (Tables 3 and 4).
Figure 1
Drug interactions and adverse drug events related to respiratory diseases, drugs, and pharmacological classes. Rename: National List of Essential Medicines; WHO: List of Essential Medicines by World Health Organization; PIM: potentially inappropriate medication.
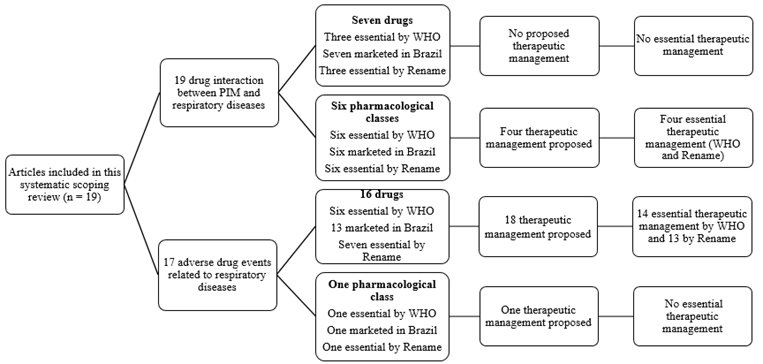
Table 3
Inappropriate pharmacological classes and drugs with concerns for elderly people with respiratory disorders according to explicit potentially inappropriate medications criteria-based tools, rationale and therapeutic management proposal.
| Drug/Pharmacological class (n of tool) (reference) | Rationale, related risks, and/or adverse drug events | Therapeutic management proposed by the tools |
| Betamethasone (1) (66) | Respiratory muscle weakness; respiratory failure; development of peptic ulcers (66). | Not reported |
| Buprenorphine (1) (70) | Dizziness, sedation; nausea, vomiting, constipation; euphoria, dysphoria, convulsions; hallucinations; respiratory depression (70). | Not reported |
| Carteolol (1) (66) | Exacerbate of respiratory disease; inducing of asthmatic attacks (66). | For asthma or COPD: selective beta-blockers for β1 receptors. Carvedilol can be used for heart failure complicated by COPD (66). |
| Clomethazole (1) (65) | Respiratory depression (65). | Adjust dose (500-1000 mg at bedtime) (65). |
| Codeine (1) (65) | Hypotension; sweating; constipation, vomiting; dizziness, sedation; and respiratory depression in treatments longer than two weeks (65). | Adjust of dose. For pain management: non-steroidal anti-inflammatory and anti-rheumatic (65). |
| Dextropropoxyphene (1) (70) | Dizziness, sedation; nausea; vomiting; constipation; euphoria, dysphoria; convulsions; hallucinations; respiratory depression (70). | Paracetamol; codeine; and ibuprofen. It is recommend the use for a short period of time and associated to a gastric protection (70). |
| Fentanyl (1) (72) | Respiratory depression (72). | Not reported |
| Hypnotics (1) (59) | Amnesia; prolonged sedation; cognitive impairment; ataxia; hypotension; falls; respiratory depression; (59). | Z-drugs (59). |
| Meperidine (1) (59) | Seizures, delirium, sedation; Respiratory depression (59). | Hydromorphone (59). |
| Methylprednisolone (1) (66) | Respiratory muscle weakness; respiratory failure; risk of development of peptic ulcers (66). | If the use is necessary, consider 40mg of prednisolone per day for five days (66). |
| Nitrofurantoin (1) (67) | Adverse pulmonary events; renal impairment; liver damage (67). | Cephalosporins; cotrimoxazole; and trimethoprim are preferable. The prescription of antibiotics should be according to your sensitivity (67). |
| Pentobarbital (1) (70) | Overdose; respiratory depression; cognitive impairment; psychiatric reactions (agitation, irritability, hallucinations, psychosis); falls and fractures (70). | Short or intermediate half-life benzodiazepines are preferable (70). |
| Phenobarbital (3) (56,59,70) | Dependence; central nervous system depression,(sedation, breath suppression); cognitive impairment; drowsiness; memory impairment; paradoxical reaction; irritability; falls and fractures; dyskinesia; ataxia; respiratory depression (56,59,70). | Other antiepileptic drugs (e.g. lamotrigine, valproic acid, levetiracetam, gabapentin). Clinical monitoring of adverse reactions (gait stability test, coordination; psychopathology) and therapeutic monitoring are recommended (70). |
| Prednisolone (1) (66) | Respiratory muscle weakness; respiratory failure; development of peptic ulcers (66). | If necessary, 40mg per day (66). |
| Propranolol (2) (65,66) | Exacerbate or cause respiratory depression; induction of asthmatic attacks; adverse events in the central nervous system (65,66). | If necessary, use three doses of 20 mg daily. Cardioselective beta-blockers; angiotensin-converting enzyme inhibitors; diuretics are recommended. For bronchial asthma or COPD: selective beta-blockers for β1 receptors. Carvedilol can be used for heart failure complicated by COPD (65,66). |
| Theophylline (2) (68,70) | Arrhythmias; adverse drug reactions due to their narrow therapeutic range; controversial efficacy in COPD patients (68,70). | Monitoring of cardiac function (70). |
| Thiopental (1) (70) | Overdose; respiratory depression; cognitive impairment, psychiatric reactions (agitation, irritability, hallucinations, psychosis); falls and fractures (70). | Lower dosage of short half-life benzodiazepine (70). |
Table 4
Essentiality of therapeutic equivalent proposed for potentially inappropriate medications for the elderly with respiratory disorders, according to National List of Essential Medicines (Rename - Brazil) and List of Essential Medicines (World Health Organization).
Discussion
In our systematic scoping review, 19 tools reported 21 drug interactions, and 17 potentially inappropriate medications (16 drugs and one pharmacological class) with concerns related to three respiratory disorders (asthma, COPD, and respiratory failure). The most frequent pharmacological classes reported were beta-blockers and benzodiazepines. The tools recommended beta-blockers management in cardiovascular disease treatment with other antihypertensive classes and short or intermediate half-life benzodiazepines.
At the beginning of the pandemic, studies suggested that angiotensin-converting enzyme inhibitors and angiotensin II type I receptor blockers could be risk factors for COVID-19 infection (24). Such hypotheses were based on the fact that high blood pressure was the most prevalent comorbidity among patients diagnosed with COVID-19 infection (25,26), and the virus binding to angiotensin-converting enzyme-2 during the infection process (27). These hypotheses were widely publicized in the media, which led some prescribers to replace angiotensin-converting enzyme inhibitors and angiotensin II type-I receptor blockers for other antihypertensive drugs, such as calcium channel blockers, due to the class is not associated with increased expression of angiotensin-converting enzyme-2 (24). Moreover, patients began to drop the treatment with angiotensin-converting enzyme inhibitors and angiotensin II type I receptor blockers on their own (28), which led to uncontrolled high blood pressure and other cardiovascular diseases, increasing the risks of COVID-19 infection (10).
However, the association between the use of angiotensin-converting enzyme inhibitors and angiotensin II type-I receptor blockers and the risk of infection by COVID-19 was not confirmed (29,30). This confusion can be justified because cardiovascular comorbidities may be linked to COVID-19 infection. Being often prescribed to treat such conditions, these pharmacological classes may be considered a confounding factor to COVID-19 infection (31).
Our systematic scoping review did not identify angiotensin-converting enzyme inhibitors and angiotensin II type I receptor blockers as potentially inappropriate medications, nor associated with adverse drug events related to respiratory disorders. Besides, these pharmacological classes are recommended by the tools for managing beta-blockers use, being considered safer options for the elderly in general and with respiratory disorders. However, studies suggest that the use of cardioselective beta-blockers in patients with COPD or asthma is not associated with severe adverse drug events (32-34). That risk can vary according to cardioselectivity, dose, and duration of the elderly's exposure to the medication (35).
From another perspective, chloroquine or hydroxychloroquine, drugs that are being widely studied for prophylaxis and COVID-19 treatment (36,37), have not been identified as potentially inappropriate medications or involved in drug interactions with respiratory disorders. However, chloroquine or hydroxychloroquine are indicated to treat rheumatological, immunological, and infectious diseases, constituting an off-label use in the infection of COVID-19. Besides, there is no evidence of high quality regarding the real risk/benefit of this use. The higher frequency of use and therapeutic overdose may be associated with significant adverse drug events (e.g., cardiomyopathy (38), prolongation of the QT interval (39), and psychiatric manifestations (e.g., depression, insomnia, and psychosis) (40)). Specifically, in Canadian elderly people with COVID-19, Ross et al. (41) identified that 58.9% were receiving one or more home medications that could potentially interact with hydroxychloroquine. Of these, 43.2% were flagged as potentially inappropriate medication by the MedSafer tool (41).
Benzodiazepines, other pharmacological classes frequently involved in drug interactions with respiratory disorders, are widely prescribed to manage anxiety and insomnia in older adults (42). Nevertheless, the long term use of benzodiazepines has been associated with a high risk of respiratory depression (43,44) and dementia, despite some uncertainty (45). The management of benzodiazepines is limited; however, the monitoring of possible adverse drug events and deprescription could be an interesting process.
Therefore, the drug therapy assessment and safety risks in the elderly with respiratory disorders are essential to promote patient security since drug interactions may reduce the effectiveness of the inhaled medications and increase the risk of adverse drug events (46). Furthermore, there are other issues to be considered, such as fewer diagnoses of respiratory disorders, difficulty in acquiring drugs for the disease control, and management in low-income patients (47), in addition to treatment adherence failures (48,49). The failure to adhere to treatment is related to several factors, difficulty in acquiring the medication, and knowledge lacking proper medication use (48). Consequently, these factors are related to uncontrolled respiratory disorders, which increases the cost of hospitalizations (e.g., mechanical ventilation due to respiratory failure (1,50)), and reduces the life quality of the elderly (47,48).
In this context, an important strategy to identify and solve drug-related problems and adherence failures would be the medication therapy management service that allows identifying possible drug-related problems and the medication experience of the patient and/or caregiver/relatives concerning the drugs in use (51). Furthermore, considering COVID-19 times, it is necessary to identify and monitor the elderly with possible worse prognosis related to COVID-19 for a targeted and specific approach that can be implemented (7).
Hence, considering the risks of using potentially inappropriate medications by older adults with respiratory disorders and the possible health problems related to adverse drug events and COVID-19, the knowledge of drug interactions and potentially inappropriate medications contributes to the adequacy of clinical monitoring, discontinuation, and deprescription of these medications (52). Still, the deprescription contributes to greater adherence to pharmacological treatment and reduces medication errors, possible adverse drug events and costs (53), thus promoting patient safety and the rational use of drugs by the elderly with respiratory disorders (54).
Our study did not aim to evaluate the correlation between potentially inappropriate medications uses and worsening of the prognosis of COVID-19; however, it highlights the importance, in COVID-19 times, of redoubling the care of the elderly with respiratory disorders and in use of potentially inappropriate medications. The assessment regarding the worsening of the COVID-19 prognosis and use of pharmacological classes and drugs identified in this review requires studies specifically designed to identify the presence or absence of this association.
One limitation of this review, as with any systematic search, is that missing studies could exist. However, manual searches were conducted to supplement electronic search limitations. In addition, our systematic scoping review excluded tools containing only implicit criteria.
Conclusion
In conclusion, 19 drug interactions between potentially inappropriate medication and respiratory disorders (asthma, chronic obstructive pulmonary disease, and respiratory failure) were reported. The beta-blockers and benzodiazepines were the most inappropriate pharmacological classes involved in the drug interactions due to the worsening of respiratory disorders and respiratory depression, increasing mucus production, and promoting bronchoconstriction concerning the antagonistic effects in B2 receptors.
Although studies initially suggested that angiotensin-converting enzyme inhibitors and angiotensin II type I receptor blockers could be associated with COVID-19 risk, such pharmacological classes are not considered potentially inappropriate medications. They are not involved in drug interactions, adverse drug events, or concerns related to respiratory disorders. Moreover, these pharmacological classes are considered safe therapeutic equivalents for the management of beta-blockers.
Therefore, the identification of drug interactions and potentially inappropriate medications, the knowledge of possible adverse drug events, and possible concerns related to the use of these drugs can collaborate to detect elderly people who may have worse prognoses of other respiratory insufficiencies and COVID-19. It can also help implement a care plan targeted and specific for the elderly with respiratory disorders, thus promoting the safety of the geriatric patient.