INTRODUCTION
According to WHO (World Health Organization), people with diabetes rose from 108 million in 1980 to 422 million in 2014, while premature mortality from diabetes increased by 5% between 2000 and 2016 (1). Due to the metabolic disorder of carbohydrates in diabetes, there is chronic exposure to hyperglycemia (2). Early diagnosis, blood sugar testing, diet, physical activity, and treatment with oral hypoglycemic agent or insulin will lead to efficient control of diabetes and prevent complications (3).
In low- and middle-income countries, medicinal plants that reduce blood sugar play an important role in the fight against this disease (4). In our lab, we have been testing the activity of Prosopis ruscifolia, Fabaceae, a plant used in folk medicine to treat diabetes. Our previous result showed the hypoglycemic effect of ethanol leaf extract in mice and rats (5,6), in addition to the hepatoprotective and nephroprotective effect in mice (7). This work reports the effect of P. ruscifolia’s extract on glycemia, insulin and incretin secretion, and various biochemical parameters in serum and urine. The experimental model was conducted using Wistar rats with alloxan- induced hyperglycemia.
MATERIAL AND METHODS
Plant material and extract preparation
Leaves of Prosopis ruscifolia Griseb. (Fabaceae), locally named viñal were collected in Presidente Hayes, Paraguay (24°23’43,9” S; 058°05’43,4” W) and a voucher with a pressed plant sample was deposited in the Herbarium of Departamento de Botánica, Facultad de Ciencias Químicas, Universidad Nacional de Asunción -UNA- (collection data S Núñez 57.135). Fresh leaves were air-dried, powdered, and extracted with ethanol by reflux method (3 times). The extract was re-dissolved in water for use in in vivo assays.
Drugs and reagents
Alloxan monohydrate from Sygma-Aldrich Co. (St. Louis, MO, USA); pentobarbital (Nembutal) from Abbott (Japan); Glucometer and strips for glucose determination in the blood from HUMAN brand, GlucoPlusTM, Canada. Kits for estimation of all biochemical parameters were purchased from HUMAN Diagnostics Worldwide reagent. The measurements were done in a Biosystem BTS 350 semi-automatic analyzer. Kits for insulin and incretin immunoassay determination were purchased from Merck Sharp & Dohme and the specific DPP-4 inhibitor (DPP-IV inhibitor) from Millipore, Canada.
Animals and ethical issues
Wistar rats, male and female, 10 to 12 weeks, and 200 to 300 g body weight from the Bioterio of Departamento de Farmacología, Facultad de Ciencias Químicas, Universidad Nacional de Asunción were used. All animals were housed under standard conditions at constant room temperature (23°C-25°C), 12:12 hours light- dark cycle, a humidity-controlled environment (50%-60%). All assays were conducted following international standards of animal welfare (8), and the research protocol was approved by the Bioethical Committee of the Facultad de Ciencias Químicas (approval code CEI 386/18).
Alloxan induced hyperglycemia and treatment
Wistar rats were randomly selected and assigned into 8 groups (n = 6). Inductions of hyperglycemia were done in four groups of animals fasted for 18 h and treated with alloxan monohydrate (110 mg/kg, intraperitoneal, i.p.) in saline solution (9). After alloxan injection, all animals received a standard pellet diet and glucose 10% solution overnight, and 48 h later, fasting blood glucose concentration was measured. Animals considered hyperglycemic were those with a blood glucose concentration higher than 180 mg/dL (10). Four groups of rats with hyperglycemia (groups Hveh, HPr50, HPr75 and HPr100) and four groups with normoglycemia (groups Nveh, NPr50, NPr75 and NPr100) received distilled water (veh, 0.1 ml/10g of weight, per os, p.o.,) or Prosopis ruscifolia (Pr) ethanolic extract (50, 75 or 100 mg/kg, p.o.) once a day for 28 days. The influence of Pr extract on glycemia was tested weekly on days 0, 7, 14, 21 and 28 (11).
Blood collection and estimation of biochemical parameters
At the end of the experiment, day 28, each rat was placed in individual metabolic cages to have accurate food and water intake records and measure the exact amount of urine produced in 24 hours. On day 30, after 6 hours of fasting, all the animals were anesthetized with sodium pentobarbital (40 mg/ Kg, i.p.), and the blood was extracted by cardiac puncture. Blood collection was performed using a serum tube and EDTA (ethylenediaminetetraacetic acid) plasma tubes. When the clot was formed, the blood was centrifuged to separate the serum, and it was fractionated to perform lipid, liver, and kidney profiles (12,13). The biochemical parameters were determined from the serum (alanine transaminase (ALT or GPT), aspartate aminotransferase (AST or GOT), alkaline phosphatase (ALP), creatinine, urea, uric acid, total protein, and albumin). Additionally, total cholesterol (TC), High-Density Lipoprotein Cholesterol (HDL-c), and triglyceride (TG) were estimated using diagnostic kits. Low-Density Lipoprotein cholesterol (LDL-c) and Very Low-Density Lipoprotein Cholesterol (VLDL-c) were calculated using Friedevald’s equation (VLDL-c =Serum triglyceride/5; LDL= TC- VLDL-c - HDL-c). Next, the anticoagulated samples were used to determine the levels of glycated hemoglobin HbA1c. Pooled 24h urine was analyzed for urea, creatinine, and uric acid levels. Results were expressed in mg/dL.
Oral glucose tolerance test and insulin and incretin level
Oral glucose tolerance test (OGTT) was performed on rats two weeks after the beginning of Prosopis ruscifolia treatment. Two groups of rats with hyperglycemia and two groups with normoglycemia (n=10) received distilled water (0.1 ml/10g of weight, p.o., Hveh and Nveh) or Pr ethanolic extract (75 mg/kg, p.o.; HPr75 and NPr75) once a day for 14 days. On day 14th, rats received orally D-glucose at 1.5 g/kg body weight (14). Blood samples were drawn from the lateral tail vein using tubes with EDTA at 0, 30, 60, 90, and 120 min after glucose administration to determine glucose in plasma, insulin, and incretin. A specific DPP-4 inhibitor (DPP-IV inhibitor, Millipore, Montreal, Canada) was immediately added to the pre-chilled EDTA tube. Immunoassays measured insulin and incretin levels in plasma (Rat Insulin ELISA kit and GLP-1 Total ELISA kit, respectively. Both kits are based on a Sandwich ELISA (15)).
Insulin determination: sample and standard (Rat Insulin Protein Standard) were added in a micro- plate that contains capture antibodies pre-coated (anti-Insulin polyclonal antibody), samples and standard linked with the antibodies after incubation and gentle shaking. Subsequently, Biotinylated Rat Insulin Detection Antibody was added. After incubation, shaking and washing; it was incubated again with HRP-Streptavidin for conjugation to the immobilized biotinylated antibody. Finally, stop solution was added (chlorhydric acid) and immediately, absorbance was read at 450 nm (15).
Incretin analysis steps: (1) capture of GLP-1 total molecules by a pre-titered amount of anti-GLP-1 polyclonal antibody, (2) binding of biotinylated anti-GLP-1 monoclonal antibody to the captured molecules, (3) conjugation of horseradish peroxidase to the immobilized biotinylated antibodies, (4) quantification of immobilized antibody-enzyme conjugated by monitoring horseradish peroxidase activities in the presence of the substrate 3,3´,5,5´-tetramethylbenzidine. The enzyme activity was measured spectrophotometrically by the increased absorbency at 450 nm, corrected from the absorbency at 590 nm, after acidification of formed products (15).
RESULTS
Effect of the ethanolic extract of P. ruscifolia on blood glucose level in normal and hyperglycemic rats
Four groups each of Wistar rats with normal glycemia (n=6) or alloxan-induced hyperglycemia (n=6) were orally treated with the extract of P. ruscifolia for 28 days. The normoglycemic animals received per group water (Nveh, 0.1mL/10g), 50 (NPr50), 75 (NPr75), and 100 (NPr100) mg/kg of the extract. These animals maintained normal glycemia, without statistically significant alterations, throughout the observation period. The hyperglycemic animals received per group water (Hveh, 0.1mL/10g), 50 (HPr50), 75 (HPr75), and 100 (HPr100) mg/kg of the extract. The hyperglycemic group treated with 75mg/kg of Pr extract significantly reduced glycemia compared to the group that received the vehicle (initial glycemia: 380.3±57.74 mg/dL, and final glycemia 125.50±1 .50).
Effect of the ethanolic extract of P. ruscifolia on several biochemical parameters in normal and hyperglycemic rats
The different biochemical parameters were measured at the end of the 28-day treatment period (Table 1). First, a statistically significant difference was observed between the glycated hemoglobin (HbA1c) values of the normoglycemic and vehicle- treated hyperglycemic rats. All hyperglycemic animals showed a significant elevation of this parameter compared to the healthy control group. Nevertheless, HbA1c remained higher than the control group after P. ruscifolia treatment despite lowering blood glucose level, probably because the experiment duration was only 28 days. Regarding cholesterol, no significant difference was observed between the plasma values of the experimental groups. However, a significant elevation of the triglyceride level was observed in the group of hyperglycemic animals treated with 50 mg/kg compared to the normoglycemic control group and the hyperglycemic control group. A non- significant increase in triglyceride was observed in the normoglycemic animals treated with the extract compared to the normoglycemic control group. HDL values were not different in any experimental group.
Table 1
Effect of the ethanolic extract of P. ruscifolia on the biochemical parameters in serum of rats treated for 28 days. Data were expressed as mean ± SD (n = 6).
Treatment: Nveh and Hveh: water. NPr50, NPr75, NPr100 (P. ruscifolia extract: 50, 75 and 100mg/kg, respectively), HPr50, HPr75, HPr100 (P. ruscifolia extract: 50, 75 and 100mg/kg, respectively). a p <0.05, b p <0.01, c p <0.001, compared with Hveh; d p <0.05, e p <0.01; f p <0.001 compared with Nveh, after Duncan test. GPT: alanine amino- transferase, GOT: aspartate aminotransferase; ALP: alkaline phosphatase.
The liver enzymes GPT, GOT, and ALP remained unchanged in the groups. However, alkaline phosphatase showed an elevation in all hyperglycemic animals compared to the normal control group; however, the difference was not significant in the groups treated with 50 and 75 mg/kg (HPr50 and HPr75). Regarding the renal profile values, an elevation of serum urea, creatinine, and uric acid was observed in the hyperglycemic animals compared with the normoglycemic. Furthermore, the plasma urea level of the hyperglycemic animals treated with the extract, HPr50, HPr75, and HPr100, were significantly lower than the hyperglycemic animals that were not treated (Hveh). A significant increase in uric acid level was also seen in the hyperglycemic animals treated with 75mg/kg (HPr75) compared to the hyperglycemic control (Hveh), this result could be due to the method used which is very cumbersome, and the wide biological variability in the animals.
Urea and creatinine levels were higher in the urine of alloxan-induced hyperglycemic animals (Hveh) than in the control group (Nveh). In the hyperglycemic group treated with 50 mg/kg both, urea, and creatinine levels were even higher, than the untreated pathological group. Uric acid was elevated in the untreated hyperglycemic group compared to the untreated normoglycemic group and it was significantly lower in the three groups of treated hyperglycemic animals versus the normoglycemic group treated with water. No changes were evidenced in total proteins or albumin levels.
Effect of P. ruscifolia on the oral glucose tolerance test and on the secretion of insulin and incretin in normal and hyperglycemic rats
The oral glucose tolerance test was done with 75 mg/kg of Pr, selected upon the extract results on glycemia, which was reduced compared to the initial glycemia and the hyperglycemic group not treated with P. ruscifolia. All the animals treated with the Pr extract showed better tolerance to oral glucose, when compared to the hyperglycemic control group, and did not differ from the control group treated with water (Figure 1).
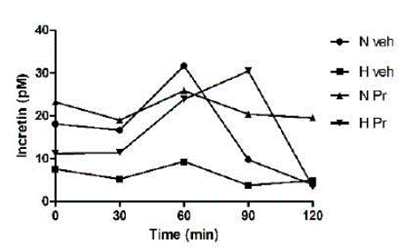
The group with normal glycemia treated with the extract (NPr) maintained uniform incretin secretion during the observed period. The pathological group always kept a low secretion level (Figure 2).
Figure 1
Effect of the extract of Prosopis ruscifolia (75 mg/kg, p.o.), in the oral glucose tolerance test, in normoglycemic and alloxan induced hyperglycemic rats (n = 6). Results are expressed as mean ± standard deviation, one-way ANOVA test and Dunnett’s post-test.
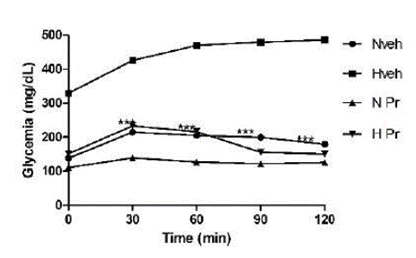
Figure 2
Effect of the extract of Prosopis ruscifolia (75 mg/kg, p.o.), in the incretin secretion, in normoglycemic and alloxan induced hyperglycemic rats (n = 6). Results are expressed as mean ± standard deviation, one-way ANOVA test and Dunnett’s post-test.
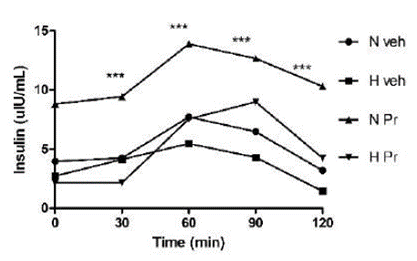
Insulin secretion in healthy animals that received the extract (NPr) was uniform and statistically different from the pathological group. After 90 minutes, it was observed that the group of hyperglycemic animals treated with the extract (HPr) showed increased insulin secretion compared to the animals in normal and hyperglycemic groups; however, it did not show a statistically significant difference (Figure 3).
Figure 3
Effect of the extract of Prosopis ruscifolia (75 mg/ kg, p.o.), in the insulin secretion, in normoglycemic and alloxan induced hyperglycemic rats (n = 6). Results are expressed as mean ± standard deviation, one-way ANOVA test and Dunnett’s post-test.
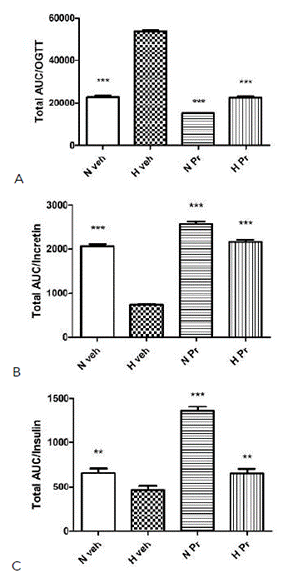
Additionally, comparing the areas under the curve (AUC, Figure 4 A, B, and C) of the three parameters measured, a marked difference between the pathological control group (Hveh) and the other experimental groups (Nveh, HPr, NPr) can be seen in the oral glucose tolerance test (Figure 4 A). This included those with normal glycemia treated with water and extract (Nveh and NPr), as well as with the extract-treated hyperglycemic group (HPr). Regarding incretin secretion, the animals that received the extract, normal glycemia or hyperglycemic, had a higher AUC of incretin than hyperglycemic control groups, with a statistically significant difference, and the values were very similar to control normoglycemic group (Figure 4 B). Finally, insulin area under the curve values was clearly and statistically higher in the healthy group (Nveh), and in the two groups treated with the extract (NPr and HPr), compared to the pathological group, Hveh (Figure 4 C). Finally, the insulin index was calculated, and the data obtained indicated a marked increase in all groups compared to the untreated hyperglycemic group (Table 2).
Figure 4
Effect of Prosopis ruscifolia (75 mg/kg, p.o.), on the area under the curve (AUC) in the oral glucose tolerance test (A), incretin (B) and insulin secretion (C) in normoglycemic and alloxan induced hyperglycemic rats (n = 10). Results are expressed as mean ± standard deviation, one-way ANOVA test, and Dunnett’s post -test. ** p< 0.01, *** p< 0.001.
DISCUSSION
As previously demonstrated, P. ruscifolia extract reduced glycemia level in hyperglycemic rats (5). The high level of triglycerides in the group of hyperglycemic rats treated with 50 mg/kg was probably due to a mistake in the measurement, considering the results of the other two treatments and the normoglycemic groups. In general terms, the ALP was elevated in the hyperglycemic animals. Diabetes mellitus affects all systems in the body, including the liver, and can lead to nonalcoholic fatty liver disease, which can further progress even until hepatocellular carcinoma. Alkaline phosphatase (ALP) has been found raised in diabetes patients compared with a non-diabetes control group (16). However, other studies found no significant association between ALP and diabetes incidence (17). The influence of liver enzymes level in diabetes remains controversial (18).
Serum creatinine and urea levels could be elevated in patients with poorly controlled blood sugar levels. An increase in blood urea level in the presence of high blood sugar levels in a diabetic patient indicates kidney damage. Previous studies demonstrated that increased urea and serum creatinine in diabetic rats indicates progressive renal impairment (19).
Insulin and glucagon secretion depends mainly on circulating glucose, but other stimuli are involved, and the effect of some hormones produced by the gastrointestinal tract is now also known (20). Incretins, such as glucagon-like peptide-1 (GLP-1) and gastric inhibitory polypeptide (GIP), are intestinal peptide hormones that enhance glucose- dependent insulin secretion after food intake. GLP-1 exerts its action through G protein-coupled receptors, highly expressed in the β cells of the islets of Langerhans. These are rapidly cleared by the kidney and inactivated by a ubiquitous exopeptidase, dipeptidyl peptidase-4 (21).
This work demonstrated that the serum incretin levels were significantly lower in the Hveh group compared to the other groups. Diabetic patients have decreased incretin levels, possibly due to several factors such as reduced GLP-1 secretion, increased incretin metabolism, or ineffective response to dietary or hormonal overload (21). Furthermore, there is evidence that subjects with diabetes do not show the incretin effect derived from oral glucose administration (20,22,23,24,25). Rats treated with 75 mg/kg of P. ruscifolia extract normalized their incretin levels. Normoglycemic rats treated with the same dose demonstrated a more sustained level of incretin secretion with oral glucose overload, suggesting that the administration of the extract stimulates sustained incretin secretion from intestinal cells.
Serum insulin secretion was also significantly lower in the hyperglycemic control group (Hveh) compared to the other experimental groups. The alloxan destroys β cells, and consequently, insulin synthesis and secretion decrease in hyperglycemic rats (9). Treatment of diabetic rats with P. ruscifolia significantly increased the insulin level compared to the Hveh group. This difference is most evident when plotting the AUC (figure 4 C). The increased insulin secretion in hyperglycemic animals treated with the extract (HPr75) suggests the activation of the remaining β cells in the pancreas of diabetic rats. Active compounds from plant with antihyperglycemic activity can act by mechanisms such as increasing repair/proliferation of pancreatic β-cells, stimulating insulin secretion, and increasing the oxidative capability (26,27,28). Furthermore, the normoglycemic rats treated with the extract (NPr75) showed a higher insulin level than all the experimental groups. This result agrees with the better glucose tolerance of the NPr75 group demonstrated by the lower glycemic levels of these animals during the experimental period (120 min). This could be due to the ability of the extract to induce insulin secretion from the regenerated beta cells (26,27,28), either by a direct stimulus or by an indirect mechanism involving incretins. With oral glucose overload a greater secretion of insulin is observed in normoglycemic rats treated with the extract (NPr75) than those receiving only the vehicle (Nveh).
Previous reports on other Prosopis species showed their ability to reduce blood glucose levels. P. cineraria demonstrated antihyperglycemic potential in mice (29) and rats (30). P. glandulosa showed effectivity increasing insulin levels and reducing blood glucose levels (31). Moreover, Soni et al. (2018) demonstrated the inhibition of α-amylase enzyme activity of the chloroform fraction of P. cineraria, and potential in vitro and in vivo antidiabetic activity, attributable to multitarget mode of action that includes antihyperglycemic, postprandial hypoglycemic, hypolipidemic, and insulin secretory actions (32). They identified in this fraction lupeol, sitosterol, and stigmasterol; some of those compounds have been reported in P. ruscifolia (5), and perhaps it can be thought that the same mechanism is responsible of the effect observed in our experiment.
Oxidative stress is one of the major determinants for the development of diabetes. Increased oxidative stress has been associated with protein unfolding and consequently the loss of protein function (33). Antioxidants in natural phytocompounds attenuate oxidative stress-mediated diabetes and improve beta-cell function, insulin secretion, and incretin-related pathways. Naringenin, for example, improved the signaling pathways that caused a surge in insulin sensitivity (34). Resveratrol causes a decline in the production of hepatic glucose (34). Prosopis ruscifolia also contains flavonoids (6), and the mechanism could be related to the antioxidant properties.
Finally, all measurements were done in male and female rats, considering that alteration due to the estrous cycle in females may not be more than a reasonable assumption. Both testosterone and estrogen are powerful neuromodulators. The same concerns should apply to hormone-associated variability in males and females (35).