Introduction
Bovine leukemia virus (BLV) is an oncogenic and lymphocytotropic Deltaretrovirus belonging to family Retroviridae, subfamily Orthoretrovirinae and genus Deltaretrovirus. It is the aetiological agent of enzootic bovine leukosis (EBL). This agent is phylogenetically related to human T-cell leukemia virus type 1 (HTLV-1; Gillet et al., 2007; Rodriguez et al., 2011). The EBL has been eradicated in many countries, mainly in the European Union (Nuotio et al., 2003; Acaite et al., 2007), but this disease is still routinely diagnosed in all Latin- American countries. Economic losses caused by EBL are mainly due to premature culling, reduction in milk production, and commercial restrictions with countries where official control has been implemented (Chi et al., 2002). The BLV induces chronic infection, usually with persistent lymphocytosis, as well as death due to lymphosarcoma in 5-10% of the infected animals. Mortality is high in cattle older than 4-5 years. BLV is mainly transmitted horizontally through indirect exposure to biological fluids containing previously infected B-lymphocytes. Colostral antibodies show high inhibitory activity until day 3 of lactation (Rodriguez et al., 2011; Konishi, et al., 2018).
The presence of horseflies, poorly sanitized dehorning procedures, colostrum feeding, artificial insemination, embryo transfer, blood transfer, and improper practices can lead to iatrogenic transmission and have been described as risk factors associated with BLV infection (Hopkins and DiGiacomo, 1997; Kobayashi et al., 2010). Therefore, eradication and control of BLV are based on early diagnosis culling, segregation, and elimination of carriers and improvement of farm management and biosecurity (Nuotio et al., 2003; Rodriguez et al., 2011; Ruggiero et al., 2019).
There are no EBL eradication programs in Ecuador and control measures are not widely applied. Therefore, this study aimed to establish individual and herd prevalence of BLV, which would increase visibility of the infection in this geographic area, and determine the risk factors associated with BLV seropositivity in dairy and dual-purpose cattle herds from Ecuador.
Materials and Methods
Study design
A cross-sectional study was carried out to determine BLV seroprevalence in Ecuador. Extensive production systems are mainly used for cattle farming in Ecuador (most farms do not have basic infrastructures; such as stables). Autochthonous/creole racial groups (e.g. Chusco, galapagueño, jaspeado manabita) or crossbred (e.g. Creole-Holstein, Jersey- Holstein) are the most abundant cattle breeds in this country. Few farms have pure breeds such as Jersey, Holstein, Ayrshire, Charolais, Brown Swiss, among others (FAO, 2020).
In these geographical and productive conditions, the study was carried out in dairy and dual-purpose (dairy-beef) farms located in the main milk-producing provinces of Ecuador (i.e., Azuay, Chimborazo, Cotopaxi, Manabí, Santo Domingo, Pichincha, Tungurahua, and Zamora Chinchipe).
Cattle older than 6 months constituted the study population, while all cattle from dual- purpose and dairy herds from Ecuador were the target population. A herd was considered as dairy or dual-purpose when milk was collected and sold.
A herd was considered positive to BLV, and therefore, infected if at least one animal was seropositive. An epidemiological software (Win Episcope 2.0) was used to calculate the sample size. A minimum sample size of 386 herds was obtained using 50% of expected prevalence (there are no previous studies in the area), a 95% confidence level, and an acceptable error of 5%.
Due to the lack of detailed cattle herd identification information in Ecuador, the following procedure for herd selection was used: First, stratified sampling was performed, according to the number of herds in each province regarding the population in all of the provinces included in the study. Second, each province was divided into blocks of twenty- five square kilometers. Third, the blocks were randomly selected. Following the order provided by the software, all of the farms in the first block were visited (cluster sampling), and then all of the farms in the second block, and so on until the total number of herds per province was obtained.
A total of 386 herds were sampled. Sample size for detecting if a herd was infected was also calculated with epidemiological software using an intra-herd prevalence of 35% (based on preliminary studies), a herd size of 10,000 (greater than the largest herd in Ecuador), and a significance level of 95%, yielding a minimum sample size of 8 animals per herd. As most herds are smaller in the study area, (>80% of the herds with <100 animals), sample size showed better sensitivity to detect infected herds (with the same number of samples, sensitivity is greater if herd size is smaller). Among farms with herd size of eight animals or less, all animals older than six months were sampled. This design has been used in similar studies in Ecuador (Saa et al., 2012).
Serological analysis
Blood samples (10 ml) from each animal were collected by puncture in the caudal vein with a needle using tubes without anticoagulant (Vacutainer®, Becton-Dickinson). Serum samples were stored at -25 ˚C until analyses.
Presence of antibodies to BLV was tested using a commercial blocking enzyme-linked immunosorbent assay (ELISA Ingezim BLV Compac2.0, Madrid, Spain) based on two monoclonal antibodies against viral gp51. This test allows detecting antibodies against BLV in bovine serum or milk (individual or pooled). Plates were coated with BLV gp51 and bound to the plate trough two specific monoclonal antibodies against gp51 virus protein. After adding the sample to the well, if it contains specific antibodies against the virus, they will bind to the antigen absorbed on plate. In contrast, if the sample does not contain specific antibodies, they will not bind to the antigen. Adding the substrate in the presence of peroxidase develops a colorimetric reaction. According to the manufacturer, diagnostic test sensitivity and specificity were 100%, so true prevalence was considered equivalent to the apparent prevalence.
Data collection
Considering variables potentially associated with BLV infection, a structured questionnaire was completed in each herd by direct interview with the farmer. Our observations were supported with feedback from the farmers. The variables included were grouped by topics: Cattle-related data, general data about the farm, management measures, reproduction system, feeding, facilities, introduction of the infection, and animal health.
Statistical analysis
An initial selection of the explanatory variables was performed using the Chi-square test to determine the risk factors associated with infection, selecting variables associated with BLV seropositivity with p<0.05. Next, a Phi test was used for dichotomic variables to determine collinearity between selected explanatory variables. If Phi was > (0.4), only the variable more logically associated with BLV seropositivity was retained (the p-values associated with Phi were <0.001 in all cases). On the other hand, an association between BLV seropositivity and quantitative variables was determined using a Kolmogorov-Smirnov test for independent variables (variables were selected when p<0.05). In a second step, correlations between independent quantitative variables were evaluated by an r of Pearson. If r was > (0.4), only the variable more logically associated with BLV seropositivity was retained.
The effect of the previously selected exploratory variables on the dependent variable was investigated using a generalized estimating equation (GEE) model. The BLV seropositivity was the dependent variable, and the herd was included as a random effect. Changes in the OR greater than 20% were considered indicative of confounding (associations could be due to a third variable associated with both the dependent and the independent variable). The model was re-run until all of the remaining variables presented with statistically significant values (the likelihood- ratio Wald’s test, p<0.05), and a potential causal relationship with the dependent variable existed. The choice of the best model was based on quasi-likelihood under the independence model criterion (QIC). The statistical analysis was performed using software (SPSS v15.0). The maps were developed with a commercial software (ArcMapTM 9.3).
Map design
To obtain the probability map of BLV seropositivity occurrence in Ecuador, a model of maximum entropy ecological niche model (MaxEnt; version 3.4.1; Phillips, 2006) was used. Similarly, to determine the regions of maximum probability of prevalence, 139 notifications of seropositivity were used, distributed in two subgroups.
Nineteen independent variables with a resolution of 1 km sourced from WorldClim were used (Hijmans et al., 2005). The model was calibrated with a default convergence threshold, regularization of 1, and a number of interactions of 5000. A logistic model was used to obtain values between 0 and 1. Those variables with high weight from the 19 climatic variables were identified, and Spearman’s coefficient was calculated to determine the correlation between variables. If the Spearman’s Rho was >0.6, one of the variables was eliminated. Finally, four variables were selected (Bio1: annual mean temperature; Bio 4: seasonality of the temperature-standard deviation; Bio12: annual rainfall; and, Bio 13: rainfall in the wettest month).
The final model was evaluated using cross- validation with 75% of the samples for training (105 notifications) and 25% for validation (34 notifications). In this way, the yields were evaluated using the area under the curve (AUC). The final model was used to generate the BLV seroprevalence map, using QGIS 2.14.
Results
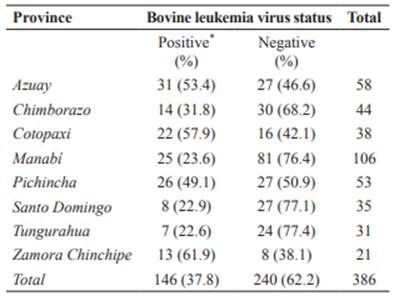
A total of 2,668 serum samples from 386 herds were taken. Individual seroprevalence of BLV was 17.3% (461/2,668; CI95% = 15.86- 18.74%), while herd prevalence was 37.8% (146/386; CI95% = 33.0-42.6%). Intra-herd prevalence ranged from 12.5 to 100% (median:37.5%).
In the univariable analysis (Chi-squared analysis), seroprevalence was significantly higher in dairy herds (20.4%) compared to dual- purpose herds (11.1%). Significantly higher seropositivity was also found in crossbred cattle (23.1%) as compared to pure breed and creole cattle jointly (14.1%; Table 1).
Table 1
Nominal variables associated (p<0.15) with bovine leukemia virus (BLV) seropositivity at herd level among dairy and dual-purpose cattle herds in Ecuador.
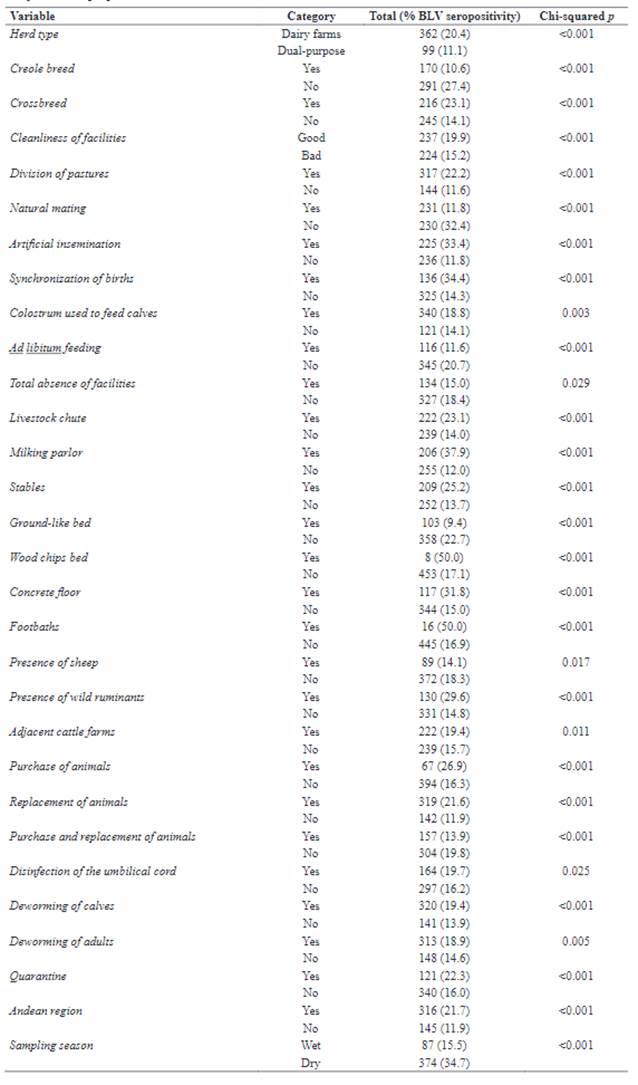
BLV seroprevalence significantly increased in herds with more than 40 animals (20.6 vs. 13.3% in herds <40 animals). The distribution of BLV seropositivity among provinces is shown in Table 2.
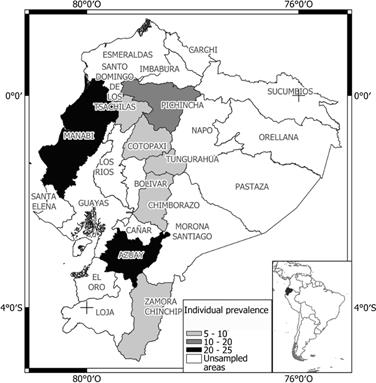
The highest seroprevalence (>50%) was found in Azuay, Cotopaxi, and Zamora Chinchipe provinces. Figures 1 and 2 show prevalence and distribution of BLV seropositivity in Ecuador at individual and herd level, respectively. Distribution of BLV seropositivity was widespread. Infection was present in all provinces, ranging from 22.6% in Tungurahua to 61.9% in Zamora Chinchipe.
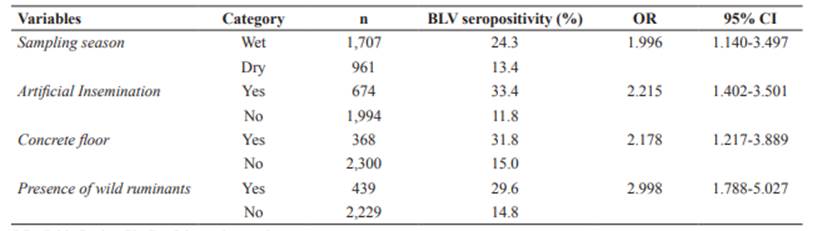
Results from the univariable analysis are shown in Tables 1 and 3. No confounding factors or potentially relevant interactions between independent variables were observed in the final model. The GEE model included sampling season (wet), artificial insemination, concrete floor, and presence of wild ruminants as risk factors associated with BLV seropositivity (Table 4).
Rainfall in the wettest month (46.9%), temperature (23.4%), annual precipitation (15.3), and temperature seasonality (14.4%) were identified as climatic factors with the greatest predictive value for occurrence of BLV seropositivity in Ecuador. The AUC of the data for training was 0.97, while that for validation was (0.888). Thus, the model agreed exceptionally well for both training and validation data.
The results show that probability of seropositivity increases for annual rainfall between 750 to 900 mm, with high monthly variations (rainfall in the wettest month between 75 to 120 mm). Mean annual temperature was between 10 to 15 degrees, ranging between 6.2 and 7.5 degrees (standard deviation; Figures 3 and 4).
Table 3
Quantitative variables selected in the univariable analysis for bovine leukemia virus (BLV) seropositivity in dairy and dual-purpose cattle farms from Ecuador (p<0.05).
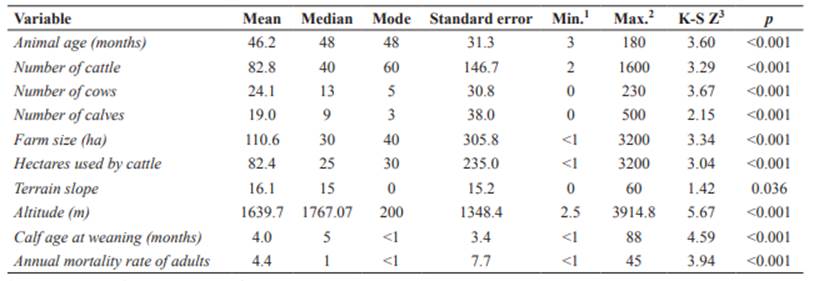
Figure 3
Spatial distribution of 139 bovine leukemia virus (BLV) cases (seroprevalence). Black dots indicate BLV seropositive cases.

Figure 4
Probability map of bovine leukemia virus (BLV) seropositivity occurrence in Ecuador. The darkest colors show increased probability of seropositivity occurrence, while the black dots indicate seropositive of BLV cases.

Discussion
This is the first nationwide survey to estimate the prevalence of antibodies and determine the risk factors associated with BLV seropositivity in Ecuador. Individual prevalence was 17.3%, agreeing with those previously reported in Brazil (7.6-50%) and Argentina (32.8%; Trono et al., 2001; Amoril et al., 2009; Rodriguez et al., 2011). Herd prevalence was 37.8%, which is lower than that described in Argentina (84%; Trono et al., 2001; Gutierrez et al., 2011). Our results are consistent with the herd prevalence of BLV (34 to 94%) found in Colombia, Venezuela, Chile, Uruguay and US (Alfonso et al., 1998; Rodriguez et al., 2011; LaDronka et al., 2018). However, herd prevalence could be underestimated because it is possible that herds with intra-herd prevalence lower than 35% were not detected as positive.
The results indicate that BLV infection is widespread among dairy and dual-purpose cattle herds in Ecuador. This may be due to poor management factors and the absence of eradication programs in this country. Another contributing factor for the high distribution of BLV infection could be the abundance of horseflies and blood-sucking insects present in the farms, especially in the warm wet season (Bech-Nielsen et al., 1978; Chi et al., 2002; Kobayashi et al., 2014). Unfortunately, no routine control against horseflies is generally practiced in Ecuador.
Associations with BLV seropositivity only by univariable analysis (such as breed) or type of herd can be caused by other factors - not the variable itself, and have been included in the results section just as descriptive results. The GEE model included AI, concrete floor, presence of wild ruminants, and season of sampling. In Ecuador, AI (OR: 2.215; CI95% = 1.402-3.501) usually implies rectal palpation (to test if the cow is already pregnant and if the reproductive tract is in good condition for an adequate pregnancy), which is usually performed without changing gloves and sleeves between animals. Although vertical transmission has been reported for leukosis virus, it is more probably that the high prevalence where AI is used be due to the mentioned inadequate practices. Our results are consistent with previous reports by Divers et al., 1995; Rodriguez et al., 2011; and Kobayashi et al., 2014.
Concrete floor was also a risk factor for BLV seropositivity in the area (OR: 2.178; CI95% = 1.217-3.889). This floor type is more abrasive than other floors in the area (e.g., pastures, ground, wood shaving), so animals could be at a higher risk of suffering wounds that would be a gateway for BLV. Therefore, cattle are enclosed, and contact with other animals is increased (Carbonero et al., 2011; Saa et al., 2012).
Improper handling practices, such as use of the same hypodermic needles for different cows, can lead to iatrogenic transmission. These practices have been described as risk factors for other infections in Ecuador (Sargeant et al., 1997; Saa et al., 2012; Carbonero et al., 2015). In addition, close contact during milking may favor BLV transmission.
Presence of wild ruminants in grazing areas was another next factor included in the model (OR: 2.998; CI95% = 1.788-5.027). Presence of wild ruminants was considered positive (yes; 16.8%; 149/386) if the farmer affirmed that wild ruminants had been observed in the farm surroundings. Species belonging to Mazama and Odocoileus genus are the most common wild deer species in the area (Tirira, 2007). Chomel et al., (1994) reported that black-tailed deer (Odocoileus hemionus) developed BLV infection under experimental conditions. However, Yokoi et al. (2009) did not find evidence of BLV infection in domestic sika deer (Cervus nipponyesoensis) in Japan. Although the role of wildlife, particularly wild ruminants, in BLV epidemiology remains unclear, this study suggests that they could play a role in the epidemiology of BLV infection. More studies are needed to clarify this point. Disease transmission between domestic and wild animals frequently results in severe consequences for the health of both species (Yokoi et al., 2009; Martin et al., 2011).
Sampling season (i.e., wet) was the other risk factor included in the model (OR: 1.996; CI95% = 1.140-3.497). Other authors have described that BLV follows a seasonal pattern, and it is related to abundant horseflies and hematophagous insects during summer, being equivalent to the hot and wet season in the sampled areas of Ecuador. The role of different bloodsucking insects in the natural transmission of BLV is well known (Bech-Nielsen et al., 1978; Hopkins et al., 1997). In this sense, high presence of horseflies has been previously associated with BLV seropositivity (Kobayashi et al., 2010; 2014). The fact that one of the frequent signs of BLV is lymphocytosis increases the probability of infection (Bech-Nielsen et al., 1978).
In conclusion, these results indicate that BLV infection is widespread in dairy and dual- purpose cattle herds in Ecuador. The BLV seroprevalence is significantly influenced by AI, use of concrete floors, sampling season (wet), and presence of wild ruminants. Considering the risk factors determined by the model, our results suggests that a control program to fight against BLV infection should be implemented in Ecuador (Hopkins et al., 1997).