Introduction
Urban development and environmental changes caused by humans have facilitated the appearance of emergent and re-emergent zoonoses, with the diseases transmitted by ticks being some of the most important (Dantas- Torres et al., 2012). The epidemiological interest in ticks as ectoparasites is due to their vectorial capacity (mechanical and biological) to transmit multiple infectious agents such as viruses, protozoans, and bacteria to wild hosts (Soares et al., 2017) and to domesticated mammals and humans (Little, 2010; Dantas-Torres and Otranto, 2011). The ability of ticks to survive in diverse ecosystems (Dantas-Torres and Otranto, 2011) and their capacity to feed on a wide range of hosts generate a worrisome scenario where they could become reservoirs for amplification or maintenance of pathogenic microorganisms (Viana et al., 2014). Regarding the hosts, stray animals in shelters come from adverse conditions that directly compromise their immune response (Shaw et al., 2017). Poor nutritional status, the absence of sanitary conditions, high ectoparasite loads, and other external stress factors may increase the susceptibility of these animals to developing infectious diseases (Rojas et al., 2013).
Although Ehrlichia canis and Hepatozoon canis are frequently seen in veterinary practice, they are poorly investigated in Colombia. Consequently, the lack of local reports about tick-borne pathogens (TBP) underestimates the importance of these pathogens in canines (Canis lupus familiaris) in our region, and the role that these domestic animals could be playing over the impact of those zoonoses on public health (Vargas-Hernández et al., 2012).
Ehrlichia canis is the etiologic agent of canine monocytic ehrlichiosis (CME) and belongs to the Anaplasmataceae family. The members of this family are Gram-negative and obligate intracellular bacteria. The CME is a multi-systemic disease that has acute, subclinical, or chronic presentations and causes a range of forms from asymptomatic to severe clinical signs characterized by depression, anorexia, lethargy, weight loss, and fever (Little, 2010). Canine hepatozoonosis is caused by two parasites from the subphylum Apicomplexa: Hepatozoon canis and H. americanum (Baneth et al., 2000). Although they are phylogenetically related, both species differ in several aspects, including clinical signs, life cycles, vectors, and host spectrum (O’Dwyer et al., 2011).
Therefore, the main objective of this study was to detect circulation of E. canis and H. canis and measure the point prevalence in Shelter dog populations from south of the Aburrá Valley with only a cursory assessment of association. Our results are highly relevant to increase alertness about the presence of E. canis and H. canis in asymptomatic dogs improving disease diagnosis and control to avoid spreading among dogs and preventing zoonotic transmission.
Materials and Methods
Ethical considerations
Field blood samples were obtained during the clinical follow-up of the patients, according to the norms stipulated by the Code of Ethics of the Professional Council of Veterinary Medicine and Zootechnics of Colombia (COMVEZCOL). The study was approved by the Ethical Committee for Animal Experimentation of Corporación Universitaria Lasallista (statement of approval 06 of June 8, 2013).
Sampling and blood collection
We conducted a cross-sectional study involving collection of blood samples and information about creole dogs in animal shelters from the municipalities of Caldas (Primavera and La Miel), La Estrella, and Sabaneta (Sabaneta and Pan de Azúcar) between July 2017 to February 2018 (Figure 1). Shelter dog population was considered because it provided defined and full access to the population of dogs from the south of the Aburrá Valley registered with the Health Secretaries of the corresponding municipalities. Accordingly, inclusion and exclusion criteria were not considered for this study.
Three hundred fifty-seven (357) blood samples were obtained from five municipal dog shelters located in the southern part of the Aburrá Valley, province of Antioquia (Colombia). Samples were collected through cephalic vein puncture in tubes with EDTA and stored at -20 oC until processing.
Nucleic acid extraction and polymerase chain reaction
The DNA was extracted from blood samples using GeneJET Genomic DNA kits (Thermo Fisher Scientific Inc., Vilnius, UAB, Lithuania), following the manufacturer's recommendations. The molecular detection of members of the Anaplasmataceae family was performed using real-time PCR (qPCR) with primers EC16SF and EC16SR (Table 1). Real-time detection was enabled using SensiFAST™ SYBR Lo- Rox Kit (Bioline© London, UK). Each reaction was amplified using 10 μl of 2x SensiFAST SYBR® One-Step mix (Meridian Life Science Inc., London, NW, UK), primers (400 mM each), 6,4 μl of Nuclease-free Water DNA (New England Biolabs Inc., Hitchin, UK) and 2 ul of DNA sample in a final volume of 20 μl. The β actin - Canis lupus familiaris (F 5’-GCGCAAGTACTCTGTGTGGAT-3’ and R 5’-GTCGTACTCCTGCTTGCTGAT-3’) was used as an internal control gene and A. marginale DNA was used as a positive control. Samples were considered positive by a melting curve between 80 to 85 °C. Positive samples were tested with conventional PCR to amplify a fragment of the 16S rRNA (468 base pairs bp) and dsb (684 bp) genes. For the detection of Hepatozoon sp., we amplified a fragment that corresponded to the gene of the subunit 18SrRNA (Table 1). DNA from H. canis infected dog blood was used as a positive control. Ultrapure water was used as a negative control.
Conventional PCR reaction was amplified using 1U of TopTaq DNA Polymerase (Qiagen, Chatsworth, CA, USA), 1x TopTaq PCR Buffer, 0.2 µM of each primer, 200 µM of each dNTP, 50∼100 ng of DNA template, and ultrapure water, in 50 μl final volume. The PCR was performed using a cycling protocol of 94 °C for 3 min, and 35 cycles of 94 °C for 30 s, 60 °C (16S rRNA and dsb) and 58 °C for 30 s (18S rRNA), and 72 °C for 1 min. Amplified products were separated on 2% agarose gel Tris Acetate-EDTA electrophoresis and visualized by staining with DNA EZ-Vision (Avantor, Inc., Bridgewater, NJ, USA).
Figure 1
Geographical distribution of the study area (and the corresponding shelters in each municipality) for the molecular detection E. canis and H. canis. The following are the coordinates for each shelter: 1. La Miel, 6°06’11’N 75°36’56’W, located at 1,855 meters above the sea leve (m.a.s.l.); 2. Primavera, 6°00’17’N 75°36’59’W, located at 2,252 m.a.s.l.; 3. La Estrella, 6°08’57’N 75°38’14’W, located at 1,655 m.a.s.l.; 4. Pan de Azúcar, 6°08’00’N 75°37’49’W, located at 1,776 m.a.s.l.; 5. Sabaneta, 6°08’39’N 75°36’45’W, located at 1,674 m.a.s.l.
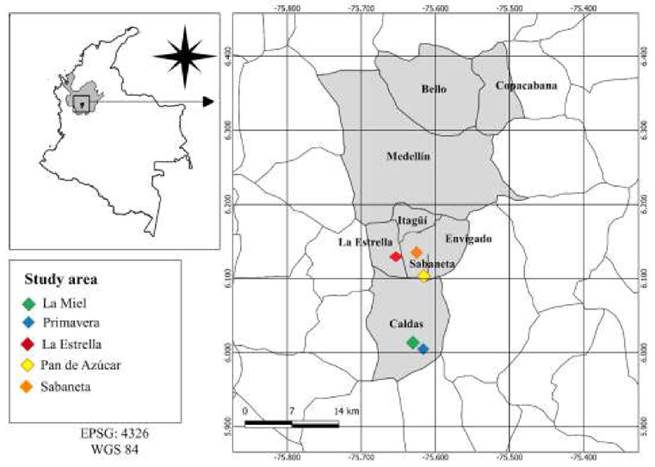
Table 1
Primers used for detecting family Anaplasmataceae and Hepatozoon sp.
| Target gene | Name | Sequence | Reference |
|---|---|---|---|
| 16S rRNA (120 bp) | EC16S F | 5'-TCGCTATTAGATGAGCCTACGT-3' | (Peleg et al., 2010; Waner et al., 2014) |
| EC16S R | 5'-GAGTCTGGACCGTATCTCAGT-3' | ||
| 16S rRNA (468 bp) | 16sANA-F | 5`-CAGAGTTTGATCCTGGCTCAGAACG-3` | (De la Fuente et al.,2006; Almazán et al., 2016) |
| 16sANA-R | 5`-AGTTTGCCGGGACTTCTTCTGTA-3` | ||
| dsb (684 bp) | dsbF2 | 5`-CTTAGTAATACTAGTGGCAAGTTTTCCAC-3` dsbR2 | (Cruz et al., 2012) |
| dsbR2 | 5'-AGGGAGCCTGAGAGACGGCTACC-3' PIRO-B | ||
| 18S rRNA (450 bp) | PIRO A1 | 5`- GTTGATATATCAGCTGCACCACCG-3` PIRO A1 | (Jefferies et al., 2003) |
| PIRO-B | 5'-TTAAATACGAATGCCCCCAAC-3' |
Statistical analysis
The analysis was based on 95% level of confidence (Zα) for measuring the point prevalence, and on 5% type I error level for detection of infection of E. canis and H. canis (dependent variable). Confidence intervals for true prevalence were calculated using a standard formula (Evans and O'Connor, 2007). Data were imported into IBM SPSS Statistics software, version # 25.0 (release 2017-03, IBM Corp., New York, USA) and descriptive analyses was undertaken (frequencies for independent categorical data). Futhermore, we performed associations between E. canis and H. canis infection with age, sex, and origin, using Pearson's chi-squared test and odds ratio (OR of prevalence).
Sequence analysis and phylogenetic tree construction
All the positive products were sequenced by Macrogen Inc. in Seoul, Korea. Nucleotide sequences for the above-mentioned gene targets were edited, assembled and trimmed using the MEGA software v6.2013 (Pennsylvania, USA) and subsequently compared with existing sequences in the GenBank database using the BLAST algorithm in NCBI (www.ncbi.nlm. nih.gov/blast). Variance estimation for pairwise analysis for all gene sequences was carried out by the bootstrap method with 1,000 replicates and uniform evolutionary rates among sites. The 16S rDNA, dsb and 18S rDNA nucleotide sequences were furtherly tested by Maximum Likelihood (ML) phylogeny. For each of the phylogenetic tree buildings we used a dataset for each of the genes. The corresponding dataset was tested in MEGA 6.0 to assess the most suitable model to generate the most reliable phylogenetic tree (Tamura et al., 2013).
Results
All positive dogs were clinically healthy. These dogs were located in 5 shelters of the 3 municipalities studied and a summary of their demographics and history is presented in Table 2.
The odds ratios (OR) calculated according to the statistical analysis did not show 95% confidence intervals (CI) that excluded 1, therefore we could not show any associations between infection of E. canis and H. canis with age, sex, or origin.
Prevalence of E. canis infection
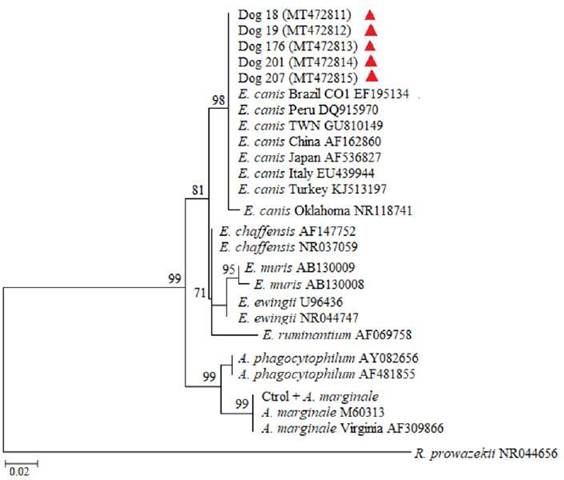
Eight dogs were positive for E. canis, representing 2.2% prevalence (IC 95% 0.27-1.72). Partial DNA fragments from 16S rRNA and/or dsb genes were amplified and the sequences obtained for both genes exhibited 100% identity with E. canis, as shown on the distributed phylogenetic clusters (Figures 2 and 3). The sequences were submitted from the GenBank and the sequence identification numbers were MT472811 to MT472820.
Table 2
Descriptive statistics for 357 dogs sampled at dog shelters in Southern Aburrá Valley during 2017 and 2018.
Figure 2
Tree of maximum likelihood and bootstrap method with 1,000 replicates based on nucleotide sequences of DNA 16S rRNA gene. The red triangles identify the sequences obtained from the dogs that tested positive in the study. Evolutive distances were calculated using the GTR + I + Γ model. The size of the aligned sequences was about 460 nt, and the tree was rooted with partial sequence of strain Brein1 16S ribosomal RNA Rickettsia prowazekii.
Prevalence of H. canis infection
Thirty-one samples were positive for H. canis (8.7% IC 95% 1.77-2.31), showing identity of 100% with sequences of genotypes from Japan (LC169075), Brazil (AY471615), and Spain (AY150067). However, Dog 6 (MT678549) sequence was related with sequences of H. canis from Brazil (MF692036), Iran (KT736298), Italy (QU371447) and Hungary (KJ572976) (Figure 4). The sequences in this study were submitted to the GenBank and the sequence identification numbers were MT472821 to MT472834.
Figure 3
Tree of maximum likelihood and bootstrap method with 1,000 replicates based on nucleotide sequences of DNA dsb gene. Evolutive distances were calculated using the GTR + I + Γ model. The size of the alignment was about 680 nt, and the tree was rooted with partial sequence of Cowdria ruminantium clone 18hw hypothetical outer membrane protein gene.
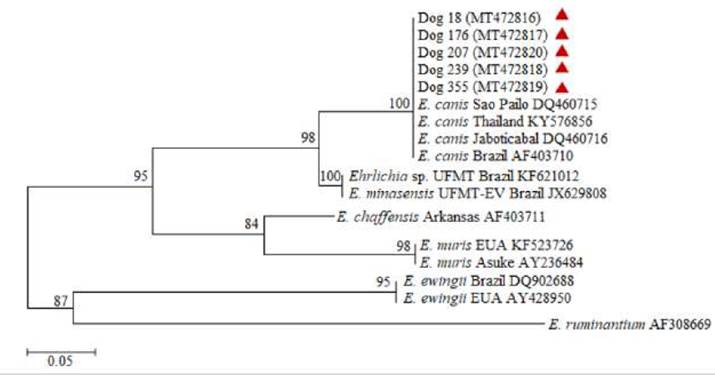
Figure 4
Tree of maximum likelihood and bootstrap method with 1,000 replicates based on nucleotide sequences of DNA 18S rRNA that show phylogenetic relationships between the apicomplexans. Blue triangles represent sequences obtained from the positive dogs. Evolutive distances were calculated using the GTR + I + Γ model. The size of the aligned sequences was about 450 nt, and the tree was rooted with Adelina sp. SH-2015 isolate HU4 18S ribosomal RNA gene.

Discussion
The TBPs cause critical infections that are potentially fatal. The present study detected E. canis and H. canis DNA in asymptomatic stray dogs. We did not find risk association for age, sex, or origin; however, previous seroprevalence studies in Brazil and Costa Rica determined that age is a risk factor associated with E. canis seropositivity; stating that the older the dog, the greater the probability of exposure to infection for TBPs (between 2-7 years (RR: 1.6, 95% CI: 1.2-2.2) and 8-15 years (RR: 1.8, 95% CI:1.2-3.0; Little, 2010; Barrantes-González, 2016).
The frequency of E. canis infection was 2.2% (8/357). This is lower than a previous study in Colombia (46 animals tested in 2016), which reported 10.9% prevalence (Posada-Zapata et al., 2017). A further survey conducted in the southwest of the country found 54% (39/72) in stray domesticated canines with and without clinical signs (Rojas et al., 2013). A 2012 study in the city of Medellin, province of Antioquia, Colombia (14 miles far from the municipalities of the present study) reported 33.3% of patients suspected to be infected with E. canis (Bonilla et al., 2012), and a 2020 study of E. canis DNA (16S rRNA and/or dsb) was detected in 18% (53/300) of dogs with clinical signs of CME by PCR amplification (Arroyave et al., 2020b). The partial sequences of 16S rRNA and dsb genes obtained in this study were consistent with previous phylogenetic analyses that show complete identity with E. canis (Waner et al., 2014; Arroyave et al., 2020b).
This is the first molecular detection of canine hepatozoonosis reported in Antioquia province, and the third report in Colombia. The first molecular detection of H. canis reported a prevalence of 31.8% in 91 dogs sheltered at Bogotá, Bucaramanga, and Villavicencio cities (Vargas-Hernández et al., 2012). After that, during the last year, we published the second molecular detection of H. canis in 350 samples of domestic dogs in Cucuta city; that study reported 8.6% frequency of infection (Chinchilla et al., 2020). Additional H. canis infections of dogs in Latin America have been reported in Mexico (Jarquín-Díaz et al., 2017), Brazil (Forlano et al., 2007; Spolidorio et al., 2009; O’Dwyer et al., 2011; Vieira et al., 2018), Argentina (Eiras et al., 2007), and Venezuela (Criado-Fornelio et al., 2007).
Primers PIRO A1 and PIRO-B have been commonly described to detect Babesia species (Jefferies et al., 2003); however, our PCR assay using these primers detected only H. canis in naturally infected dogs. Furthermore, the genetic sequence of each sample and their phylogenetic analysis confirmed this finding, suggesting that the same set of primers can be used to amplify species of the apicomplexan parasites. However, the evaluation and validity of this diagnostic test are beyond the scope of this study. Molecular studies utilizing 18S rRNA gene have allowed to identify Hepatozoon spp. circulation and analysis of the phylogenetic divergence associated with its bio-geographic distribution (Vieira et al., 2018).
E. canis is recognized as the most important tick-borne pathogen in Latin America (Dantas- Torres et al., 2012), however, other tick-borne pathogens could be in the shadow of canine ehrlichiosis. In addition, both pathogens are present in asymptomatic or severely ill dogs that show similar clinical signs such as lethargy, weight loss, cachexia, and anemia that might confuse the diagnosis (Little, 2010; Demoner et al., 2013). All of the above, along with the low sensitivity of the buffy coat smear examination and the lack of a more accurate diagnostic tests, such as serological or molecular assays, may be underestimating the true importance of H. canis.
H. canis and E. canis are transmitted by the same vector, the brown dog tick, R. sanguineus s.l, which causes that the prevalence of H. canis overlaps in areas where canine ehrlichiosis is endemic (de Miranda et al., 2014). Despite geographic and environmental conditions, no evidence of tick infestation was found on the dogs during sample collection; however, a previous study reported that R. sanguineus s.l. was the main arthropod found in dogs from Medellín city with clinical signs compatible with canine ehrlichiosis (Arroyave et al., 2020a).
In conclusion, this report confirms previous observations and epidemiological surveys from Colombia and other tick-endemic countries which show evidence of infection with E. canis and H. canis in stray dogs (Chinchilla et al., 2020). In addition, we observed that H. canis is the principal canine pathogen transmitted by ticks in the studied area, and its prevalence is in fact higher than expected, what suggests that H. canis may be commonly underreported (or underdiagnosed) in similar circumstances. Taken together, these results emphasize the need to test for multiple zoonotic TBPs in sheltered dogs and increase awareness about their likely threat to public health. Further investigation is needed to examine the genetic diversity of pathogenic microorganisms such as bacteria and eukaryotic agents in the blood of intermediate hosts, such as domesticated canines. Further research will also be required to infer the pathogenic relationships of the parasite with its natural host, and the risk of horizontal transmission where conditions for the presence of the vector are favorable. The analysis of these results is important for obtaining an epidemiological profile of the agents that silently circulate throughout the country where clinical symptoms are not obvious in dogs.