Degradación fotocatalítica de Fenol, Catecol e Hidroquinona sobre nanomateriales Au-ZnO
DOI:
https://doi.org/10.17533/udea.redin.20190513Palabras clave:
compuestos fenólicos, fotocatálisis, Au-ZnOResumen
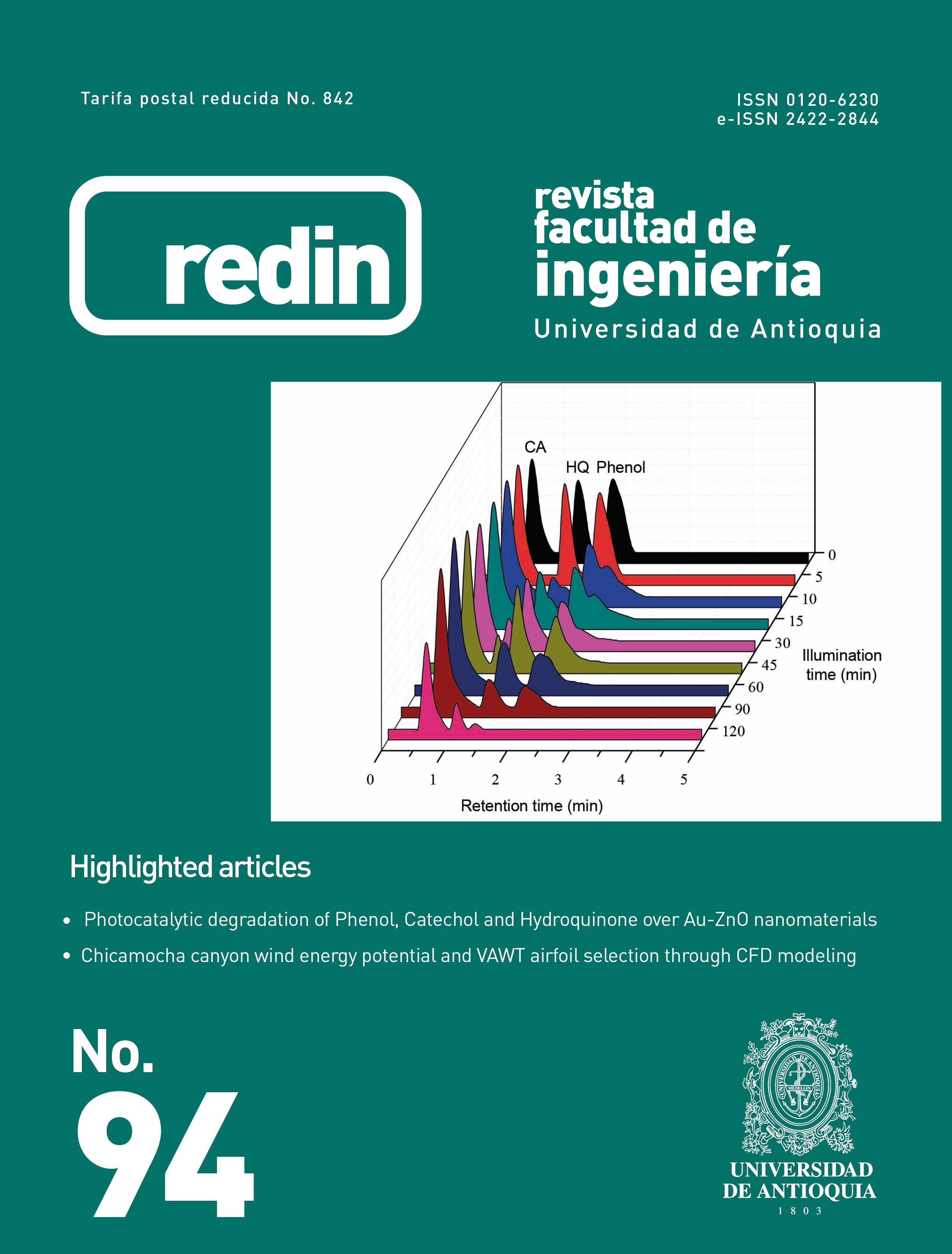
Algunos nanomateriales tipo Au-ZnO fueron evaluados en reacciones de fotodegradación bajo luz UV-Visible; Fenol, Catecol e hidroquinona fueron las moléculas modelo, en general se encontró que la Hidroquinona es la molécula más sensible a la degradación bajo iluminación. La adición de Au incrementa significativamente la actividad fotocatalítica del ZnO en la degradación de los compuestos fenólicos y el contenido de Au es un factor con una influencia muy importante sobre las propiedades físicoquímicas de los nanomateriales sintetizados y sobre la efectividad del tratamiento fotocatalítico. La efectividad más alta en la eliminación de los compuestos fenólicos se logró usando ZnO modificado por adición de 2% en peso de oro, esto se debe a la mayor absorción que presenta este material en la región visible del espectro electromagético. Por HPLC, se encontró que la ruta de degradación de los compuestos fenólicos depende del fotocatalizador empleado en la reacción y del sustrato a degradar, así, la degradación del Fenol se lleva a cabo a través de la formación de un mayor número de compuestos intermediarios que lo observado en la degradación de Catecol e Hidroquinona.
Descargas
Citas
S. Ahmed, M.G. Rasul, W.N. Martens, R. Brown, M.A. Hashi, “Heterogeneous photocatalytic degradation of phenols in wastewater: A review on current status and developments,” Desalination, Vol. 261, pp.3–18, 2010.
P. Calza, D. Vione, C. Minero, “The role of humic and fulvic acids in the phototransformation of phenolic compounds in seawater,” Sci. Total Environ., Vol. 493, pp. 411–418, 2014.
Y. Piña, F. Tzompantzi, R. Pérez, R. Arroyo, P. Acevedo, R. Gómez, “Photocatalytic activity of Al2O3 improved by the addition of Ce3+/Ce4+ synthesized by the sol-gel method. Photodegradation of phenolic compounds using UV light,” Fuel, Vol. 198, pp. 11–21, 2017.
N.H. Salah, M. Bouhelassa, S. Bekkouche, A. Boultif, “A Study of photocatalytic degradation of phenol,” Desalination, Vol. 166, pp. 347–354, 2004.
L.F. Velasco, B. Tsyntsarski, B. Petrova, T. Budinova, N. Petrova, J.B. Parra, C.O. Ania, “Carbon foams as catalyst supports for phenol photodegradation,” J. Hazard. Mater., Vol. 184, pp. 843–848, 2010.
A. Tolosana, J.A. Anderson, J.A. Casas, M. Faraldos, A. Bahamonde, “Defining the role of substituents on adsorption and photocatalytic degradation of phenolic compounds,” J Environ Chem Eng, Vol. 5, pp. 4612–4620, 2017.
M.C. Hidalgo, J.J. Murcia, J.A. Navío, G. Colón, “Photodeposition of gold on titanium dioxide for photocatalytic phenol oxidation,” Appl. Catal., A, Vol. 397, pp. 112–120, 2011.
D. Satoca, R. Gómez, “A photoelectrochemical and spectroscopic study of phenol and catechol oxidation on titanium dioxide nanoporous electrodes,” Electrochim. Acta, Vol. 55, pp. 4661– 4668, 2010.
J. Tashkhourian, M. Daneshi, F. Nami-Ana, M. Behbahani, A. Bagheri, “Simultaneous determination of hydroquinone and catechol at gold nanoparticles mesoporous silica modified carbon paste electrode,” J. Hazard. Mater., Vol. 318, pp. 117–124, 2016.
M. Su, P. Chen, Y. Dong, H. Sun, “Chemiluminescence of Graphene quantum dots induced by acidic potassium permanganate and its application to quenchometric flow-injection assays of hydroquinone in water,” J. Lumin., Vol. 177, pp. 204–208, 2016.
S. Jeenpadiphat, I. Mongkolpichayarak, D. Tungasmita, “Catechol production from lignin by Al-doped mesoporous silica catalytic cracking,” J. Anal. Appl. Pyrolysis., Vol. 121, pp. 318–328, 2016.
J.J. Murcia, J.R. Guarín, A.C. Cely Macías, H. Rojas, J.A. Cubillos, M.C. Hidalgo, J.A. Navío, “Methylene blue degradation over M-TiO2 photocatalysts (M= Au or Pt),” Ciencia en Desarrollo, Vol. 8, pp. 109–117, 2017.
C. Jaramillo-Páez, J.A. Navío, M.C. Hidalgo, M. Macías, “High UV-photocatalytic activity of ZnO and Ag/ZnO synthesized by a facile method,” Catal. Today., Vol. 284, pp. 121–128, 2017.
V. Vaiano, M. Matarangolo, J.J. Murcia, H. Rojas, J.A. Navío, M.C. Hidalgo, “Enhanced photocatalytic removal of phenol from aqueous solutions using ZnO modified with Ag,” Appl. Catal., B., Vol. 225, pp. 197–206, 2018.
G. Ahmed, M. Hanif, L. Zhao, M. Hussain, J. Khan, Z. Liu, “Defect engineering of ZnO nanoparticles by graphene oxide leading to enhanced visible light photocatalysis,” J. Mol. Catal. A: Chem., Vol. 425, pp. 310–321, 2016.
E. Friehs, Y. AlSalka, R. Jonczyk, A. Lavrentieva, A. Jochums, J. Walter, F. Stahl, T. Scheper, D. Bahnemann, “Toxicity, phototoxicity and biocidal activity of nanoparticles employed in photocatalysis,” J. Photochem. Photobiol. C., Vol. 29, pp. 1–28, 2016.
P. Georgiev, N. Kaneva, A. Bojinova, K. Papazova, K. Mircheva, K. Balashev, “Effect of gold nanoparticles on the photocatalytic efficiency of ZnO films,” Colloids Surf., A., Vol. 460, pp. 240–247, 2014.
M.M. Hasan, R.K. Srivastava, “Structural, optical and photoconductivity study of ZnO nanoparticles synthesized by annealing of ZnS nanoparticles,” J. Alloys Compd., Vol. 691, pp. 275–286, 2017.
C.G. Silva, M.J. Sampaio, S.A.C. Carabineiro, J.W. Oliveira, D.L. Baptista, R. Bacsa, B.F. Machado, P. Serp, J.L. Figueiredo, A.M. Silva, J.L. Faria, “Developing highly active photocatalysts: Gold-loaded ZnO for solar phenol oxidation,” J. Catal., Vol. 316, pp. 182–190, 2014.
Z. Guo, R. Ma, G. Li, “Degradation of phenol by nanomaterial TiO2 in wastewater,” Chem. Eng. J., Vol. 119, pp. 55–59, 2006.
K. Raja, P.S. Ramesh, D. Geetha, “Structural, FTIR and photoluminescence studies of Fe doped ZnO nanopowder by co-precipitation method,” Spectrochim Acta A Mol Biomol Spectrosc., Vol. 131, pp. 183–188, 2014.
K.-H. Wang, Y.-H. Hsieh, M.-Y. Chou, C.-Y. Chang, “Photocatalytic degradation of 2-chloro and 2-nitrophenol by titanium dioxide suspensions in aqueous solution,” Appl. Catal. B., Vol. 21, pp. 1–8, 1999.
C. Wu, X. Liu, D. Wei, J. Fan, L. Wang, “Photosonochemical degradation of phenol in water,” Water Res., Vol. 35, pp. 3927–3933, 2001.
S. Li, Z. Ma, J. Zhang, Y. Wu, Y. Gong, “A comparative study of photocatalytic degradation of phenol of TiO2 and ZnO in the presence of manganese dioxides,” Catal. Today., Vol. 139, pp. 109–112, 2008.
C. Karunakaran, R. Dhanalakshmi, “Semiconductor-catalyzed degradation of phenols with sunlight,” Sol. Energy Mater. Sol. Cells., Vol. 92, pp. 1315–1321, 2008.
Descargas
Publicado
Cómo citar
Número
Sección
Licencia
Derechos de autor 2020 Revista Facultad de Ingeniería Universidad de Antioquia

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-CompartirIgual 4.0.
Los artículos disponibles en la Revista Facultad de Ingeniería, Universidad de Antioquia están bajo la licencia Creative Commons Attribution BY-NC-SA 4.0.
Eres libre de:
Compartir — copiar y redistribuir el material en cualquier medio o formato
Adaptar : remezclar, transformar y construir sobre el material.
Bajo los siguientes términos:
Reconocimiento : debe otorgar el crédito correspondiente , proporcionar un enlace a la licencia e indicar si se realizaron cambios . Puede hacerlo de cualquier manera razonable, pero no de ninguna manera que sugiera que el licenciante lo respalda a usted o su uso.
No comercial : no puede utilizar el material con fines comerciales .
Compartir igual : si remezcla, transforma o construye a partir del material, debe distribuir sus contribuciones bajo la misma licencia que el original.
El material publicado por la revista puede ser distribuido, copiado y exhibido por terceros si se dan los respectivos créditos a la revista, sin ningún costo. No se puede obtener ningún beneficio comercial y las obras derivadas tienen que estar bajo los mismos términos de licencia que el trabajo original.









 Twitter
Twitter