Inmunorregulación inducida por helmintos: una actualización
DOI:
https://doi.org/10.17533/udea.iatreia.v29n2a07Palabras clave:
ascaris lumbricoides, helmintiasi, inmunomodulación, inmunosupresiónResumen
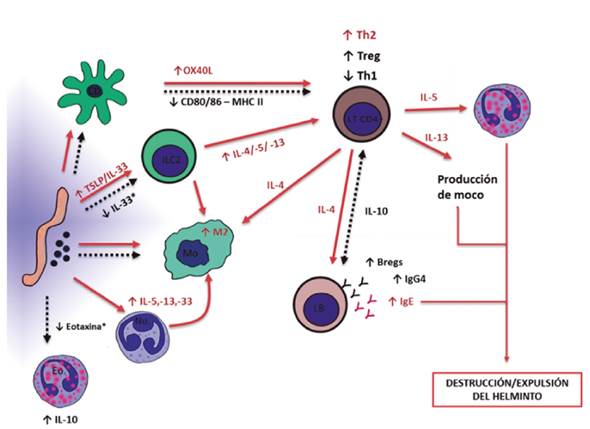
Las helmintiasis, que han desaparecido o disminuido en las regiones más desarrolladas del mundo, producen cambios notables en el sistema inmunológico, y usualmente, cuando son crónicas o intensas, causan inmunosupresión. Aunque deterioran la salud, también parecen proteger del desarrollo de enfermedades inflamatorias crónicas al proveer estímulos inmunorreguladores. Promueven el desarrollo de linfocitos B o T reguladores que inhiben la proliferación de clones autorreactivos o específicos de alérgeno. Cada vez se conoce más la modulación de la respuesta innata por parásitos; se destaca que además de aumentar en número células claves en la defensa contra estos, también pueden usarlas como blanco de evasión. Algunas poblaciones importantes en la defensa contra bacterias y otros patógenos también responden a los helmintos, pero sufren una programación genética diferente a las formas asociadas a la respuesta tipo 1. Paralelamente a la inmunosupresión, las helmintiasis inducen una respuesta tipo 2. Por esto, es preocupante lo que sucede en algunas poblaciones en donde se controlan parcialmente estas parasitosis y predominan los ciclos de infestación ligera/reinfección. En estos contextos, algunos helmintos, como Ascaris lumbricoides, parecen promover el desarrollo de alergias; esta es la helmintiasis más común en el planeta, pero de la que menos se sabe sobre su capacidad inmunorreguladora.
Descargas
Citas
(1.) Haahtela T, Holgate S, Pawankar R, Akdis CA, Benjaponpitak S, Caraballo L, et al. The biodiversity hypothesis and allergic disease: world allergy organization position statement. World Allergy Organ J. 2013 Jan;6(1):3. DOI 10.1186/1939-4551-6-3.
(2.) Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989 Nov;299(6710):1259-60.
(3.) Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. 1986. J Immunol. 2005 Jul;175(1):5-14.
(4.) Greenwood BM. Autoimmune diseases in Nigerians. Lancet. 1968 Sep;2(7567):573.
(5.) Petrillo MG, Ronchetti S, Ricci E, Alunno A, Gerli R, Nocentini G, et al. GITR+ regulatory T cells in the treatment of autoimmune diseases. Autoimmun Rev. 2015 Feb;14(2):117-26. DOI 10.1016/j.autrev.2014.10.011.
(6.) Pullan RL, Smith JL, Jasrasaria R, Brooker SJ. Global numbers of infection and disease burden of soil transmitted helminth infections in 2010. Parasit Vectors. 2014 Jan;7:37. DOI 10.1186/1756-3305-7-37.
(7.) Mkhize-Kwitshana ZL, Mabaso ML. The neglected triple disease burden and interaction of helminths, HIV and tuberculosis: an opportunity for integrated action in South Africa. S Afr Med J. 2014 Apr;104(4):258-9.
(8.) Borkow G, Bentwich Z. Chronic parasite infections cause immune changes that could affect successful vaccination. Trends Parasitol. 2008 Jun;24(6):243-5. DOI 10.1016/j.pt.2008.02.009.
(9.) Caraballo L, Acevedo N. Allergy in the tropics: the impact of cross-reactivity between mites and ascaris. Front Biosci (Elite Ed). 2011 Jan;3:51-64.
(10.) Metenou S, Dembele B, Konate S, Dolo H, Coulibaly SY, Coulibaly YI, et al. At homeostasis filarial infections have expanded adaptive T regulatory but not classical Th2 cells. J Immunol. 2010 May;184(9):5375-82. DOI 10.4049/jimmunol.0904067.
(11.) Hussaarts L, van der Vlugt LE, Yazdanbakhsh M, Smits HH. Regulatory B-cell induction by helminths: implications for allergic disease. J Allergy Clin Immunol. 2011 Oct;128(4):733-9. DOI 10.1016/j.jaci.2011.05.012.
(12.) Ricci ND, Fiúza JA, Bueno LL, Cançado GG, Gazzinelli-Guimarães PH, Martins VG, et al. Induction of CD4(+) CD25(+)FOXP3(+) regulatory T cells during human hookworm infection modulates antigen-mediated lymphocyte proliferation. PLoS Negl Trop Dis. 2011 Nov;5(11):e1383. DOI 10.1371/journal.pntd.0001383.
(13.) Doetze A, Satoguina J, Burchard G, Rau T, Löliger C, Fleischer B, et al. Antigen-specific cellular hyporesponsiveness in a chronic human helminth infection is mediated by T(h)3/T(r)1-type cytokines IL-10 and transforming growth factor-beta but not by a T(h)1 to T(h)2 shift. Int Immunol. 2000 May;12(5):623-30.
(14.) Wammes LJ, Hamid F, Wiria AE, Wibowo H, Sartono E, Maizels RM, et al. Regulatory T cells in human lymphatic filariasis: stronger functional activity in microfilaremics. PLoS Negl Trop Dis. 2012;6(5):e1655. DOI 10.1371/journal.pntd.0001655.
(15.) Taylor MD, Harris A, Babayan SA, Bain O, Culshaw A, Allen JE, et al. CTLA-4 and CD4+ CD25+ regulatory T cells inhibit protective immunity to filarial parasites in vivo. J Immunol. 2007 Oct;179(7):4626-34.
(16.) Taylor MD, LeGoff L, Harris A, Malone E, Allen JE, Maizels RM. Removal of regulatory T cell activity reverses hyporesponsiveness and leads to filarial parasite clearance in vivo. J Immunol. 2005 Apr;174(8):4924-33.
(17.) Grainger JR, Smith KA, Hewitson JP, McSorley HJ, Harcus Y, Filbey KJ, et al. Helminth secretions induce de novo T cell Foxp3 expression and regulatory function through the TGF-β pathway. J Exp Med. 2010 Oct;207(11):2331-41. DOI 10.1084/jem.20101074.
(18.) Sawant DV, Gravano DM, Vogel P, Giacomin P, Artis D, Vignali DA. Regulatory T cells limit induction of protective immunity and promote immune pathology following intestinal helminth infection. J Immunol. 2014 Mar;192(6):2904-12. DOI 10.4049/jimmunol.1202502.
(19.) Tang CL, Lei JH, Wang T, Lu SJ, Guan F, Liu WQ, et al. Effect of CD4+ CD25+regulatory T cells on the immune evasion of Schistosoma japonicum. Parasitol Res. 2011 Feb;108(2):477-80. DOI 10.1007/s00436-010-2089-2.
(20.) D’Elia R, Behnke JM, Bradley JE, Else KJ. Regulatory T cells: a role in the control of helminth-driven intestinal pathology and worm survival. J Immunol. 2009 Feb;182(4):2340-8. DOI 10.4049/jimmunol.0802767.
(21). Teixeira-Carvalho A, Martins-Filho OA, Peruhype-Magalhães V, Silveira-Lemos D, Malaquias LC, Oliveira LF, et al. Cytokines, chemokine receptors, CD4+CD25HIGH+ T-cells and clinical forms of human schistosomiasis. Acta Trop. 2008 Nov-Dec;108(2-3):139-49. DOI 10.1016/j.actatropica.2008.04.010.
(22.) Lawson CA, Brown AK, Bejarano V, Douglas SH, Burgoyne CH, Greenstein AS, et al. Early rheumatoid arthritis is associated with a deficit in the CD4+CD25high regulatory T cell population in peripheral blood. Rheumatology (Oxford). 2006 Oct;45(10):1210-7.
(23.) Lee JH, Noh J, Noh G, Choi WS, Cho S, Lee SS. Allergen-specific transforming growth factor-β-producing CD19+CD5+ regulatory B-cell (Br3) responses in human late eczematous allergic reactions to cow’s milk. J Interferon Cytokine Res. 2011 May;31(5):441-9. DOI 10.1089/jir.2010.0020.
(24.) Noh J, Choi WS, Noh G, Lee JH. Presence of Foxp3-expressing CD19(+)CD5(+) B Cells in Human Peripheral Blood Mononuclear Cells: Human CD19(+)CD5(+) Foxp3(+)Regulatory B Cell (Breg). Immune Netw. 2010 Dec;10(6):247-9. DOI 10.4110/in.2010.10.6.247.
(25.) van der Vlugt LE, Mlejnek E, Ozir-Fazalalikhan A, Janssen Bonas M, Dijksman TR, Labuda LA, et al. CD24(hi)CD27(+) B cells from patients with allergic asthma have impaired regulatory activity in response to lipopolysaccharide. Clin Exp Allergy. 2014 Apr;44(4):517-28. DOI 10.1111/cea.12238.
(26.) Blair PA, Noreña LY, Flores-Borja F, Rawlings DJ, Isenberg DA, Ehrenstein MR, et al. CD19(+)CD24(hi) CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity. 2010 Jan;32(1):129-40. DOI 10.1016/j.immuni.2009.11.009.
(27.) Khoder A, Sarvaria A, Alsuliman A, Chew C, Sekine T, Cooper N, et al. Regulatory B cells are enriched within the IgM memory and transitional subsets in healthy donors but are deficient in chronic GVHD. Blood. 2014 Sep;124(13):2034-45. DOI 10.1182/blood-2014-04-571125.
(28.) van der Vlugt LE, Labuda LA, Ozir-Fazalalikhan A, Lievers E, Gloudemans AK, Liu KY, et al. Schistosomes induce regulatory features in human and mouse CD1d(hi) B cells: inhibition of allergic inflammation by IL-10 and regulatory T cells. PLoS One. 2012;7(2):e30883. DOI 10.1371/journal.pone.0030883.
(29.) Correale J, Farez M, Razzitte G. Helminth infections associated with multiple sclerosis induce regulatory B cells. Ann Neurol. 2008 Aug;64(2):187-99. DOI 10.1002/ana.21438.
(30.) van der Vlugt LE, Zinsou JF, Ozir-Fazalalikhan A, Kremsner PG, Yazdanbakhsh M, Adegnika AA, et al. Interleukin 10 (IL-10)-producing CD1dhi regulatory B cells from Schistosoma haematobium-infected individuals induce IL-10-positive T cells and suppress effector T-cell cytokines. J Infect Dis. 2014 Oct;210(8):1207-16. DOI 10.1093/infdis/jiu257.
(31.) Iwata Y, Matsushita T, Horikawa M, Dilillo DJ, Yanaba K, Venturi GM, et al. Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood. 2011 Jan;117(2):530-41. DOI 10.1182/blood-2010-07-294249.
(32.) Rodgers DT, Pineda MA, McGrath MA, Al-Riyami L, Harnett W, Harnett MM. Protection against collageninduced arthritis in mice afforded by the parasitic worm product, ES-62, is associated with restoration of the levels of interleukin-10-producing B cells and reduced plasma cell infiltration of the joints. Immunology. 2014 Mar;141(3):457-66. DOI 10.1111/imm.12208.
(33.) McKenzie AN. Type-2 innate lymphoid cells in asthma and allergy. Ann Am Thorac Soc. 2014 Dec;11 Suppl 5:S263-70. DOI 10.1513/AnnalsATS.201403-097AW.
(34.) Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010 Apr;464(7293):1367-70. DOI 10.1038/nature08900.
(35.) Oliphant CJ, Hwang YY, Walker JA, Salimi M, Wong SH, Brewer JM, et al. MHCII-mediated dialog between group 2 innate lymphoid cells and CD4(+) T cells potentiates type 2 immunity and promotes parasitic helminth expulsion. Immunity. 2014 Aug;41(2):283-95. DOI 10.1016/j.immuni.2014.06.016.
(36.) McSorley HJ, Blair NF, Smith KA, McKenzie AN, Maizels RM. Blockade of IL-33 release and suppression of type 2 innate lymphoid cell responses by helminth secreted products in airway allergy. Mucosal Immunol. 2014 Sep;7(5):1068-78. DOI 10.1038/mi.2013.123.
(37.) Bartemes KR, Kephart GM, Fox SJ, Kita H. Enhanced innate type 2 immune response in peripheral blood from patients with asthma. J Allergy Clin Immunol. 2014 Sep;134(3):671-678.e4. DOI 10.1016/j.jaci.2014.06.024.
(38.) Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CG, Doering TA, et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol. 2011 Nov;12(11):1045-
DOI 10.1031/ni.2131.
(39.) Boyd A, Ribeiro JM, Nutman TB. Human CD117 (cKit)+innate lymphoid cells have a discrete transcriptional profile at homeostasis and are expanded during filarial infection. PLoS One. 2014 Sep;9(9):e108649. DOI 10.1371/journal.pone.0108649.
(40.) Oeser K, Schwartz C, Voehringer D. Conditional IL-4/IL-13-deficientinata mice reveal a critical role of innate immune cells for protective immunity against gastrointestinal helminths. Mucosal Immunol. 2015 May;8(3):672-82. DOI 10.1038/mi.2014.101.
(41.) Caraballo L, Zakzuk J. Consideraciones sobre la evolución de la respuesta inmunitaria Th2 y sus posibles relaciones con parasitosis y alergia. Biomédica. 2012 Jun-Mar;32(1):145-57. DOI 10.1590/S0120-41572012000100017.
(42.) Huh JY, Park YJ, Ham M, Kim JB. Crosstalk between adipocytes and immune cells in adipose tissue inflammation and metabolic dysregulation in obesity. Mol Cells. 2014 May;37(5):365-71. DOI 10.14348/molcells.2014.0074.
(43.) Balic A, Harcus Y, Holland MJ, Maizels RM. Selective maturation of dendritic cells by Nippostrongylus brasiliensis-secreted proteins drives Th2 immune responses. Eur J Immunol. 2004 Nov;34(11):3047-59.
(44.) Semnani RT, Sabzevari H, Iyer R, Nutman TB. Filarial antigens impair the function of human dendritic cells during differentiation. Infect Immun. 2001 Sep;69(9):5813-22.
(45.) van Die I, van Vliet SJ, Nyame AK, Cummings RD, Bank CM, Appelmelk B, et al. The dendritic cellspecific C-type lectin DC-SIGN is a receptor for Schistosoma mansoni egg antigens and recognizes the glycan antigen Lewis x. Glycobiology. 2003 Jun;13(6):471-8.
(46.) van der Kleij D, Latz E, Brouwers JF, Kruize YC, Schmitz M, Kurt-Jones EA, et al. A novel host-parasite lipid cross-talk. Schistosomal lyso-phosphatidylserine activates toll-like receptor 2 and affects immune polarization. J Biol Chem. 2002 Dec;277(50):48122-9.
(47.) Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014 Mar;6:13. DOI 10.12703/P6-13.
(48.) Babu S, Kumaraswami V, Nutman TB. Alternatively activated and immunoregulatory monocytes in human filarial infections. J Infect Dis. 2009 Jun;199(12):1827-37. DOI 10.1086/599090.
(49.) Reese TA, Wakeman BS, Choi HS, Hufford MM, Huang SC, Zhang X, et al. Coinfection. Helminth infection reactivates latent γ-herpesvirus via cytokine competition at a viral promoter. Science. 2014 Aug;345(6196):573-7. DOI 10.1126/science.1254517.
(50.) Chen F, Wu W, Millman A, Craft JF, Chen E, Patel N, et al. Neutrophils prime a long-lived effector macrophage phenotype that mediates accelerated helminth expulsion. Nat Immunol. 2014 Oct;15(10):938-46. DOI 10.1038/ni.2984.
(51.) Klion AD, Nutman TB. The role of eosinophils in host defense against helminth parasites. J Allergy Clin Immunol. 2004 Jan;113(1):30-7.
(52.) Huang L, Gebreselassie NG, Gagliardo LF, Ruyechan MC, Lee NA, Lee JJ, et al. Eosinophil-derived IL-10 supports chronic nematode infection. J Immunol. 2014 Oct;193(8):4178-87. DOI 10.4049/jimmunol.1400852.
(53.) Culley FJ, Brown A, Conroy DM, Sabroe I, Pritchard DI, Williams TJ. Eotaxin is specifically cleaved by hookworm metalloproteases preventing its action in vitro and in vivo. J Immunol. 2000 Dec;165(11):6447-53.
(54.) Hagan P, Blumenthal UJ, Dunn D, Simpson AJ, Wilkins HA. Human IgE, IgG4 and resistance to reinfection with Schistosoma haematobium. Nature. 1991 Jan;349(6306):243-5.
(55.) Dunne DW, Butterworth AE, Fulford AJ, Kariuki HC, Langley JG, Ouma JH, et al. Immunity after treatment of human schistosomiasis: association between IgE antibodies to adult worm antigens and resistance to reinfection. Eur J Immunol. 1992 Jun;22(6):1483-94.
(56.) Acevedo N, Erler A, Briza P, Puccio F, Ferreira F, Caraballo L. Allergenicity of Ascaris lumbricoides tropomyosin and IgE sensitization among asthmatic patients in a tropical environment. Int Arch Allergy Immunol. 2011;154(3):195-206. DOI 10.1159/000321106.
(57.) Kobayashi Y, Ishizaki S, Nagashima Y, Shiomi K. Ani s 1, the major allergen of Anisakis simplex: purification by affinity chromatography and functional expression in Escherichia coli. Parasitol Int. 2008 Sep;57(3):314-9. DOI 10.1016/j.parint.2008.01.005.
(58.) Da Silva CA, Pochard P, Lee CG, Elias JA. Chitin particles are multifaceted immune adjuvants. Am J Respir Crit Care Med. 2010 Dec;182(12):1482-91. DOI 10.1164/rccm.200912-1877OC.
(59.) Zakzuk J, Benedetti I, Fernández-Caldas E, Caraballo L. The influence of chitin on the immune response to the house dust mite allergen Blo T 12. Int Arch Allergy Immunol. 2014;163(2):119-29. DOI 10.1159/000356482.
(60.) Jeannin P, Lecoanet S, Delneste Y, Gauchat JF, Bonnefoy JY. IgE versus IgG4 production can be differentially regulated by IL-10. J Immunol. 1998 Apr;160(7):3555-61.
(61.) Bindon CI, Hale G, Brüggemann M, Waldmann H. Human monoclonal IgG isotypes differ in complement activating function at the level of C4 as well as C1q. J Exp Med. 1988 Jul;168(1):127-42.
(62.) van der Neut Kolfschoten M, Schuurman J, Losen M, Bleeker WK, Martínez-Martínez P, Vermeulen E, et al. Anti-inflammatory activity of human IgG4 antibodies by dynamic Fab arm exchange. Science. 2007 Sep;317(5844):1554-7.
(63.) Demeure CE, Rihet P, Abel L, Ouattara M, Bourgois A, Dessein AJ. Resistance to Schistosoma mansoni in humans: influence of the IgE/IgG4 balance and IgG2 in immunity to reinfection after chemotherapy. J Infect Dis. 1993 Oct;168(4):1000-8.
(64.) Adjobimey T, Hoerauf A. Induction of immunoglobulin G4 in human filariasis: an indicator of immunoregulation. Ann Trop Med Parasitol. 2010 Sep;104(6):455-64. DOI 10.1179/136485910X12786389891407.
(65.) Dold C, Holland CV. Ascaris and ascariasis. Microbes Infect. 2011 Jul;13(7):632-7. DOI 10.1016/j.micinf.2010.09.012.
(66.) Cooper PJ, Chico M, Sandoval C, Espinel I, Guevara A, Levine MM, et al. Human infection with Ascaris lumbricoides is associated with suppression of the interleukin-2 response to recombinant cholera toxin B subunit following vaccination with the live oral cholera vaccine CVD 103-HgR. Infect Immun. 2001 Mar;69(3):1574-80.
(67.) Buendía E, Zakzuk J, Mercado D, Alvarez A, Caraballo L. The IgE response to Ascaris molecular components is associated with clinical indicators of asthma severity. World Allergy Organ J. 2015 Mar;8(1):8. DOI 10.1186/s40413-015-0058-z.
(68.) Zakzuk J, Acevedo N, Cifuentes L, Bornacelly A, Sánchez J, Ahumada V, et al. Early life IgE responses in children living in the tropics: a prospective analysis. Pediatr Allergy Immunol. 2013 Dec;24(8):788-97. DOI 10.1111/pai.12161.
(69.) Palmer LJ, Celedón JC, Weiss ST, Wang B, Fang Z, Xu X. Ascaris lumbricoides infection is associated with increased risk of childhood asthma and atopy in rural China. Am J Respir Crit Care Med. 2002 Jun;165(11):1489-93.
(70.) Lynch NR, Hagel IA, Palenque ME, Di Prisco MC, Escudero JE, Corao LA, et al. Relationship between helminthic infection and IgE response in atopic and nonatopic children in a tropical environment. J Allergy Clin Immunol. 1998 Feb;101(2 Pt 1):217-21.
(71.) Araujo Z, Giampietro F, Rivas-Santiago B, Luna-Herrera J, Wide A, Clark W, et al. Patients exposed to Mycobacterium tuberculosis infection with a prominent IgE response. Arch Med Res. 2012 Apr;43(3):225-32. DOI 10.1016/j.arcmed.2012.04.002.
(72.) Cooper PJ, Chico ME, Rodrigues LC, Ordonez M, Strachan D, Griffin GE, et al. Reduced risk of atopy among school-age children infected with geohelminth parasites in a rural area of the tropics. J Allergy Clin Immunol. 2003 May;111(5):995-1000.
(73.) Figueiredo CA, Barreto ML, Rodrigues LC, Cooper PJ, Silva NB, Amorim LD, et al. Chronic intestinal helminth infections are associated with immune hyporesponsiveness and induction of a regulatory network. Infect Immun. 2010 Jul;78(7):3160-7. DOI 10.1128/IAI.01228-09.
(74.) McConchie BW, Norris HH, Bundoc VG, Trivedi S, Boesen A, Urban JF Jr, et al. Ascaris suum-derived products suppress mucosal allergic inflammation in an interleukin-10-independent manner via interference with dendritic cell function. Infect Immun. 2006 Dec;74(12):6632-41.
(75.) Rocha FA, Leite AK, Pompeu MM, Cunha TM, Verri WA Jr, Soares FM, et al. Protective effect of an extract from Ascaris suum in experimental arthritis models. Infect Immun. 2008 Jun;76(6):2736-45. DOI 10.1128/IAI.01085-07.
(76.) Itami DM, Oshiro TM, Araujo CA, Perini A, Martins MA, Macedo MS, et al. Modulation of murine experimental asthma by Ascaris suum components. Clin Exp Allergy. 2005 Jul;35(7):873-9.
(77.) Acevedo N, Mohr J, Zakzuk J, Samonig M, Briza P, Erler A, et al. Proteomic and immunochemical characterization of glutathione transferase as a new allergen of the nematode Ascaris lumbricoides. PLoS One. 2013 Nov;8(11):e78353. DOI 10.1371/journal.pone.0078353.
(78.) de Araújo CA, Perini A, Martins MA, Macedo MS, Macedo-Soares MF. PAS-1, an Ascaris suum protein, modulates allergic airway inflammation via CD8+γδTCR+ and CD4+CD25+FoxP3+ T cells. Scand J Immunol. 2010 Dec;72(6):491-503. DOI 10.1111/j.1365-3083.2010.02465.x.
Publicado
Cómo citar
Número
Sección
Licencia
Derechos de autor 2016 Iatreia

Esta obra está bajo una licencia internacional Creative Commons Atribución-CompartirIgual 4.0.
Los artículos publicados en la revista están disponibles para ser utilizados bajo la licencia Creative Commons, específicamente son de Reconocimiento-NoComercial-CompartirIgual 4.0 Internacional.
Los trabajos enviados deben ser inéditos y suministrados exclusivamente a la Revista; se exige al autor que envía sus contribuciones presentar los formatos: presentación de artículo y responsabilidad de autoría completamente diligenciados.