Activation mechanisms of invariant natural killer T cells (iNKTs)
DOI:
https://doi.org/10.17533/udea.iatreia.v29n1a05Keywords:
α-galactosylceramide, antigen presentation, CD1d, iNKT cells, glycolipids, TCRAbstract
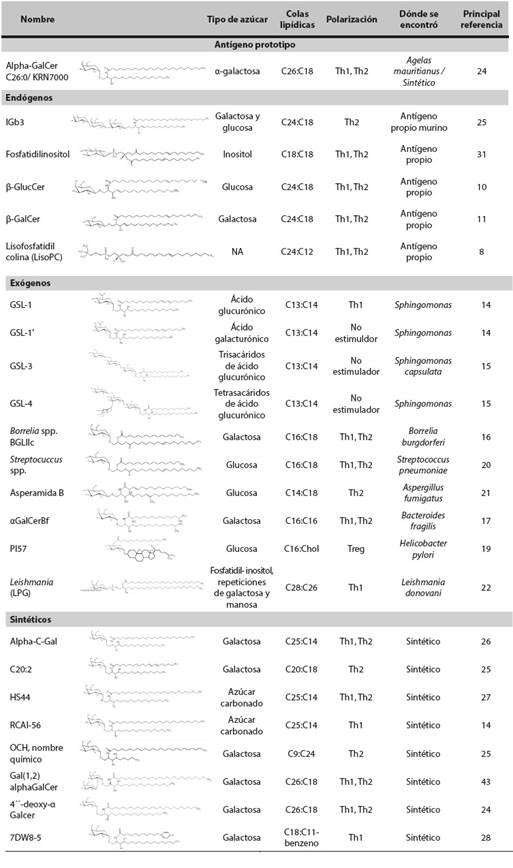
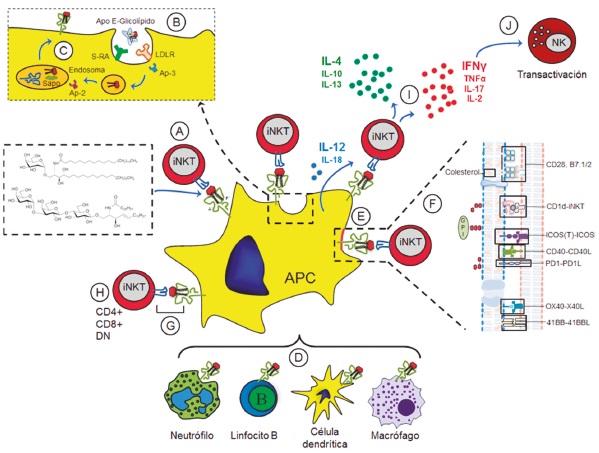
A great amount of knowledge on natural killer T cells (iNKTs) is now available, but a consensus about their activation mechanisms has not been reached. These cells recognize different glycolipid antigens through the CD1d molecule. Such antigens may be endogenous, derived from bacteria (foreign) and synthetic, the latter have been developed for clinical applications. There exists much interest in understanding how these different glycolipid compounds induce different types of polarization, but it has been difficult to reach a consensus due to the fact that responses depend on different factors such as: the nature of the molecule, the internalization process and the presentation of the glycolipids. Moreover, activation of iNKT cells is determined by the type and state of the antigen presenting cell, the co-stimulatory molecules, the transactivation mechanisms and the location of the glycolipid-CD1d complexes on the plasma membrane, such as the lipid rafts. This review explores the evidence about the factors that affect activation of iNKT cells in order to understand their immune-modulatory potential.
Downloads
References
(1.) Brennan PJ, Brigl M, Brenner MB. Invariant natural killer T cells: an innate activation scheme linked to diverse effector functions. Nat Rev Immunol. 2013 Feb;13(2):101-17. DOI 10.1038/nri3369.
(2.) Salio M, Silk JD, Jones EY, Cerundolo V. Biology of CD1- and MR1-restricted T cells. Annu Rev Immunol. 2014;32:323-66. DOI 10.1146/annurev-immunol-032713-120243.
(3.) Godfrey DI, Kronenberg M. Going both ways: immune regulation via CD1d-dependent NKT cells. J Clin Invest. 2004 Nov;114(10):1379-88.
(4.) Behar SM, Porcelli SA. CD1-restricted T cells in host defense to infectious diseases. Curr Top Microbiol Immunol. 2007;314:215-50.
(5.) Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297-336. DOI 10.1146/annurev.immunol.25.022106.141711.
(6.) Carreño LJ, Kharkwal SS, Porcelli SA. Optimizing NKT cell ligands as vaccine adjuvants. Immunotherapy. 2014;6(3):309-20. DOI 10.2217/imt.13.175.
(7.) Venkataswamy MM, Porcelli SA. Lipid and glycolipid antigens of CD1d-restricted natural killer T cells. Semin Immunol. 2010 Apr;22(2):68-78. DOI 10.1016/j.smim.2009.10.003.
(8.) Rossjohn J, Pellicci DG, Patel O, Gapin L, Godfrey DI. Recognition of CD1d-restricted antigens by natural killer T cells. Nat Rev Immunol. 2012 Dec;12(12):845-57. DOI 10.1038/nri3328.
(9.) Cox D, Fox L, Tian R, Bardet W, Skaley M, Mojsilovic D, et al. Determination of cellular lipids bound to human CD1d molecules. PLoS One. 2009;4(5):e5325. DOI 10.1371/journal.pone.0005325.
(10.) Joyce S, Woods AS, Yewdell JW, Bennink JR, De Silva AD, Boesteanu A, et al. Natural ligand of mouse CD1d1: cellular glycosylphosphatidylinositol. Science. 1998 Mar;279(5356):1541-4.
(11.) López-Sagaseta J, Sibener LV, Kung JE, Gumperz J, Adams EJ. Lysophospholipid presentation by CD1d and recognition by a human Natural Killer T-cell receptor. EMBO J. 2012 Apr;31(8):2047-59. DOI 10.1038/emboj.2012.54.
(12.) Christiansen D, Milland J, Mouhtouris E, Vaughan H, Pellicci DG, McConville MJ, et al. Humans lack iGb3 due to the absence of functional iGb3-synthase: implications for NKT cell development and transplantation. PLoS Biol. 2008 Jul;6(7):e172. DOI 10.1371/journal.pbio.0060172.
(13.) Brennan PJ, Tatituri RV, Brigl M, Kim EY, Tuli A, Sanderson JP, et al. Invariant natural killer T cells recognize lipid self antigen induced by microbial danger signals. Nat Immunol. 2011 Oct;12(12):1202-11. DOI 10.1038/ni.2143.
(14.) Inafuku M, Li C, Kanda Y, Kawamura T, Takeda K, Oku H, et al. Beta-glucosylceramide administration (i.p.) activates natural killer T cells in vivo and prevents tumor metastasis in mice. Lipids. 2012 Jun;47(6):581-91. DOI 10.1007/s11745-012-3666-1.
(15.) Fischer K, Scotet E, Niemeyer M, Koebernick H, Zerrahn J, Maillet S, mannoside is a natural antigen for CD1drestricted T cells. Proc Natl Acad Sci USA. 2004 Jul;101(29):10685-90.
(16.) Kinjo Y, Wu D, Kim G, Xing GW, Poles MA, Ho DD, et al. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature. 2005 Mar;434(7032): 520-5.
(17.) Anderson BL, Teyton L, Bendelac A, Savage PB. Stimulation of natural killer T cells by glycolipids. Molecules. 2013 Dec;18(12):15662-88. DOI 10.3390/molecules181215662.
(18.) Long X, Deng S, Mattner J, Zang Z, Zhou D, McNary N, et al. Synthesis and evaluation of stimulatory properties of Sphingomonadaceae glycolipids. Nat Chem Biol. 2007 Sep;3(9):559-64.
(19.) Kinjo Y, Tupin E, Wu D, Fujio M, Garcia-Navarro R, Benhnia MR, et al. Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat Immunol. 2006 Sep;7(9):978-86.
(20.) Wieland Brown LC, Penaranda C, Kashyap PC, Williams BB, Clardy J, Kronenberg M, et al. Production of α-galactosylceramide by a prominent member of the human gut microbiota. PLoS Biol. 2013 Jul;11(7):e1001610. DOI 10.1371/journal.pbio.1001610.
(21.) An D, Oh SF, Olszak T, Neves JF, Avci FY, Erturk-Hasdemir D, et al. Sphingolipids from a symbiotic microbe regulate homeostasis of host intestinal natural killer T cells. Cell. 2014 Jan;156(1-2):123-33. DOI 10.1016/j.cell.2013.11.042.
(22.) Ito Y, Vela JL, Matsumura F, Hoshino H, Tyznik A, Lee H, et al. Helicobacter pylori cholesteryl α-glucosides contribute to its pathogenicity and immune response by natural killer T cells. PLoS One. 2013 Dec;8(12):e78191. DOI 10.1371/journal.pone.0078191.
(23.) Kinjo Y, Illarionov P, Vela JL, Pei B, Girardi E, Li X, et al. Invariant natural killer T cells recognize glycolipids from pathogenic Gram-positive bacteria. Nat Immunol. 2011 Sep;12(10):966-74. DOI 10.1038/ni.2096.
(24.) Albacker LA, Chaudhary V, Chang YJ, Kim HY, Chuang YT, Pichavant M, et al. Invariant natural killer T cells recognize a fungal glycosphingolipid that can induce airway hyperreactivity. Nat Med. 2013 Oct;19(10):1297-304. DOI 10.1038/nm.3321.
(25.) Amprey JL, Im JS, Turco SJ, Murray HW, Illarionov PA, Besra GS, et al. A subset of liver NK T cells is activated during Leishmania donovani infection by CD1d-bound lipophosphoglycan. J Exp Med. 2004 Oct;200(7):895-904.
(26.) Wun KS, Cameron G, Patel O, Pang SS, Pellicci DG, Sullivan LC, et al. A molecular basis for the exquisite CD1d-restricted antigen specificity and functional responses of natural killer T cells. Immunity. 2011 Mar;34(3):327-39. DOI 10.1016/j.immuni.2011.02.001.
(27.) Yu ED, Girardi E, Wang J, Mac TT, Yu KO, Van Calenbergh S, et al. Structural basis for the recognition of C20:2-αGalCer by the invariant natural killer T cell receptor-like antibody L363. J Biol Chem. 2012 Jan;287(2):1269-78. DOI 10.1074/jbc.M111.308783.
(28.) Patel O, Cameron G, Pellicci DG, Liu Z, Byun HS, Beddoe T, et al. NKT TCR recognition of CD1d-α-C-galactosylceramide. J Immunol. 2011 Nov;187(9):4705-13. DOI 10.4049/jimmunol.1100794.
(29.) Kerzerho J, Yu ED, Barra CM, Alari-Pahissa E, Girardi E, Harrak Y, et al. Structural and functional characterization of a novel nonglycosidic type I NKT agonist with immunomodulatory properties. J Immunol. 2012 Mar;188(5):2254-65. DOI 10.4049/jimmunol.1103049. Erratum in: J Immunol. 2012 Oct;189(8):4194.
(30.) Li X, Fujio M, Imamura M, Wu D, Vasan S, Wong CH, et al. Design of a potent CD1d-binding NKT cell ligand as a vaccine adjuvant. Proc Natl Acad Sci USA. 2010 Jul;107(29):13010-5. DOI 10.1073/pnas.1006662107.
(31.) Barral DC, Brenner MB. CD1 antigen presentation: how it works. Nat Rev Immunol. 2007 Dec;7(12):929-41.
(32.) Xia F, Li R, Wang C, Yang S, Tian L, Dong H, et al. IRGM1 regulates oxidized LDL uptake by macrophage via actin-dependent receptor internalization during atherosclerosis. Sci Rep. 2013;3:1867. DOI 10.1038/srep01867.
(33.) Freigang S, Landais E, Zadorozhny V, Kain L, Yoshida K, Liu Y, et al. Scavenger receptors target glycolipids for natural killer T cell activation. J Clin Invest. 2012 Nov;122(11):3943-54. DOI 10.1172/JCI62267.
(34.) Florence WC, Bhat RK, Joyce S. CD1d-restricted glycolipid antigens: presentation principles, recognition logic and functional consequences. Expert Rev Mol Med. 2008 Jul;10:e20. DOI 10.1017/S1462399408000732.
(35.) van den Elzen P, Garg S, León L, Brigl M, Leadbetter EA, Gumperz JE, et al. Apolipoprotein-mediated pathways of lipid antigen presentation. Nature. 2005 Oct;437(7060):906-10.
(36.) Chen X, Wang X, Keaton JM, Reddington F, Illarionov PA, Besra GS, et al. Distinct endosomal trafficking requirements for presentation of autoantigens and exogenous lipids by human CD1d molecules. J Immunol. 2007 May;178(10):6181-90.
(37.) Salio M, Ghadbane H, Dushek O, Shepherd D, Cypen J, Gileadi U, et al. Saposins modulate human invariant Natural Killer T cells self-reactivity and facilitate lipid exchange with CD1d molecules during antigen presentation. Proc Natl Acad Sci USA. 2013 Dec;110(49):E4753-61. DOI 10.1073/pnas.1310050110.
(38.) Kang SJ, Cresswell P. Regulation of intracellular trafficking of human CD1d by association with MHC class II molecules. EMBO J. 2002 Apr;21(7):1650-60.
(39.) Vomhof-DeKrey EE, Yates J, Leadbetter EA. Invariant NKT cells provide innate and adaptive help for B cells. Curr Opin Immunol. 2014 Jun;28:12-7. DOI 10.1016/j.coi.2014.01.007.
(40.) Peng W, Martaresche C, Escande-Beillard N, Cedile O, Reynier-Vigouroux A, Boucraut J. Influence of lipid rafts on CD1d presentation by dendritic cells. Mol Membr Biol. 2007 Sep-Dec;24(5-6):475-84.
(41.) Murai T. The role of lipid rafts in cancer cell adhesion and migration. Int J Cell Biol. 2012;2012:763283. DOI 10.1155/2012/763283.
(42.) Im JS, Arora P, Bricard G, Molano A, Venkataswamy MM, Baine I, et al. Kinetics and cellular site of glycolipid loading control the outcome of natural killer T cell activation. Immunity. 2009 Jun;30(6):888-98. DOI 10.1016/j.immuni.2009.03.022.
(43.) Williams JA, Lumsden JM, Yu X, Feigenbaum L, Zhang J, Steinberg SM, et al. Regulation of thymic NKT cell development by the B7-CD28 costimulatory pathway. J Immunol. 2008 Jul;181(2):907-17.
(44.) van den Heuvel MJ, Garg N, Van Kaer L, Haeryfar SM. NKT cell costimulation: experimental progress and therapeutic promise. Trends Mol Med. 2011 Feb;17(2):65-77. DOI 10.1016/j.molmed.2010.10.007.
(45.) Singh AK, Gaur P, Das SN. Natural killer T cell anergy, co-stimulatory molecules and immunotherapeutic interventions. Hum Immunol. 2014 Mar;75(3):250-60. DOI 10.1016/j.humimm.2013.12.004.
(46.) Adams EJ, López-Sagaseta J. The immutable recognition of CD1d. Immunity. 2011 Mar;34(3):281-3. DOI 10.1016/j.immuni.2011.03.006.
(47.) Pei B, Vela JL, Zajonc D, Kronenberg M. Interplay between carbohydrate and lipid in recognition of glycolipid antigens by natural killer T cells. Ann N Y Acad Sci. 2012 Apr;1253:68-79. DOI 10.1111/j.1749-6632.2011.06435.x.
(48.) Mallevaey T, Clarke AJ, Scott-Browne JP, Young MH, Roisman LC, Pellicci DG, et al. A molecular basis for NKT cell recognition of CD1d-self-antigen. Immunity. 2011 Mar;34(3):315-26. DOI 10.1016/j.immuni.2011.01.013.
(49.) Patel O, Pellicci DG, Uldrich AP, Sullivan LC, Bhati M, McKnight M, et al. Vβ2 natural killer T cell antigen receptor-mediated recognition of CD1d-glycolipid antigen. Proc Natl Acad Sci USA. 2011 Nov;108(47):19007-12. DOI 10.1073/pnas.1109066108.
(50.) Arora P, Baena A, Yu KO, Saini NK, Kharkwal SS, Goldberg MF, et al. A single subset of dendritic cells controls the cytokine bias of natural killer T cell responses to diverse glycolipid antigens. Immunity. 2014 Jan;40(1):105-16. DOI 10.1016/j.immuni.2013.12.004.
(51.) Watarai H, Sekine-Kondo E, Shigeura T, Motomura Y, Yasuda T, Satoh R, et al. Development and function of invariant natural killer T cells producing T(h)2- and T(h)17-cytokines. PLoS Biol. 2012 Feb;10(2):e1001255.DOI 10.1371/journal.pbio.1001255.
(52.) McDonald BD, Constantinides MG, Bendelac A. Polarized effector programs for innate-like thymocytes. Nat Immunol. 2013 Nov;14(11):1110-1. DOI 10.1038/ni.2739.
(53.) Venken K, Decruy T, Aspeslagh S, Van Calenbergh S, Lambrecht BN, Elewaut D. Bacterial CD1d-restricted glycolipids induce IL-10 production by human regulatory T cells upon cross-talk with invariant NKT cells. J Immunol. 2013 Sep;191(5):2174-83. DOI 10.4049/jimmunol.1300562.
(54.) Wu J, Yang J, Yang K, Wang H, Gorentla B, Shin J, et al. iNKT cells require TSC1 for terminal maturation and effector lineage fate decisions. J Clin Invest. 2014 Apr;124(4):1685-98. DOI 10.1172/JCI69780.
(55.) King IL, Fortier A, Tighe M, Dibble J, Watts GF, Veerapen N, et al. Invariant natural killer T cells direct B cell responses to cognate lipid antigen in an IL-21-dependent manner. Nat Immunol. 2011 Nov;13(1):44-50. DOI 10.1038/ni.2172.
(56.) Chang PP, Barral P, Fitch J, Pratama A, Ma CS, Kallies A, et al. Identification of Bcl-6-dependent follicular helper NKT cells that provide cognate help for B cell responses. Nat Immunol. 2011 Nov;13(1):35-43. DOI 10.1038/ni.2166.
(57.) De Santo C, Arscott R, Booth S, Karydis I, Jones M, Asher R, et al. Invariant NKT cells modulate the suppressive activity of IL-10-secreting neutrophils differentiated with serum amyloid A. Nat Immunol. 2010 Nov;11(11):1039-46. DOI 10.1038/ni.1942.
(58.) Bai L, Constantinides MG, Thomas SY, Reboulet R, Meng F, Koentgen F, et al. Distinct APCs explain the cytokine bias of α-galactosylceramide variants in vivo. J Immunol. 2012 Apr 1;188(7):3053-61. DOI 10.4049/jimmunol.1102414.
(59.) Oki S, Tomi C, Yamamura T, Miyake S. Preferential T(h)2 polarization by OCH is supported by incompetent NKT cell induction of CD40L and following production of inflammatory cytokines by bystander cells in vivo. Int Immunol. 2005 Dec;17(12):1619-29.
Published
How to Cite
Issue
Section
License
Copyright (c) 2015 Iatreia

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
Papers published in the journal are available for use under the Creative Commons license, specifically Attribution-NonCommercial-ShareAlike 4.0 International.
The papers must be unpublished and sent exclusively to the Journal Iatreia; the author uploading the contribution is required to submit two fully completed formats: article submission and authorship responsibility.