Genotoxic and Mutagenic Assessment Induced by Vinasse, Before and After Being Subjected to Bio-oxidation and Fenton Processes.
DOI:
https://doi.org/10.17533/udea.vitae.v31n2a357688Keywords:
Vinasse, bioethanol, lymphocytes, comet assay, bio-oxidationAbstract
Background: Colombia is joining global initiatives to mitigate climate change through bioethanol production, as it has large sugar cane plantations and sugar mills, particularly in the Valle del Cauca region. One of the main by-products of the bioethanol industry is vinasse, which consists mainly of water, organic solids and heavy metals. Some of the compounds present in vinasses, such as melanoidins and phthalates, show genotoxic, mutagenic and carcinogenic activity in onion cells, tilapia and aquatic organisms. Various methods, such as bio-oxidation and Fenton reaction, have been used to reduce the organic load of vinasses. Among the most commonly used assays to study genotoxicity and mutagenicity are single cell gel electrophoresis (comet assay) and the Ames test.
Objective: In this study, the genotoxicity in human lymphocytes and the mutagenicity in Salmonella typhimurium induced by different dilutions of vinasse produced at the bioethanol production plant in Frontino, Antioquia, before and after being subjected to biooxidation and Fenton processes, were evaluated.
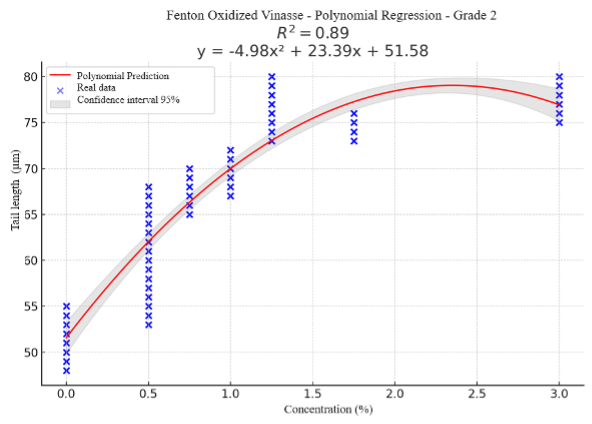
Methods: Genotoxicity was evaluated by the comet assay in human lymphocytes, and mutagenic activity was evaluated by the Ames test using Salmonella typhimurium strains TA98 and TA100, with and without the addition of microsomal enzymes (S9). Both tests were applied to each type of vinasse considered in this study, including raw vinasse (RV), bio-oxidised vinasse (BV) and Fenton oxidised vinasse (FV). Results: The results showed that at RV doses above 3%, viability decreased to values between 70% and 88%, whereas for BV and FV, viability remained above 93% and 94%, respectively. Vinasse was also found to have a dose-dependent effect on genotoxicity. However, no mutagenic activity was observed in any of the Salmonella strains evaluated, indicating that vinasse does not induce mutations.
Conclusion: The importance of addressing vinasse pollution and treatment methods to reduce its toxicity is emphasised. However, further research is needed to fully understand the risks associated with vinasse exposure and to develop effective mitigation strategies.
Downloads
References
Sydney EB, Letti LA, Karp S G, Sydney AN, Vandenberghe LP, Carvalho JC, Woiciechowski AL, Medeiros PB, Soccol VT, Soccol CR. Current analysis and future perspective of reduction in worldwide greenhouse gases emissions by using first and second generation bioethanol in the transportation sector, Bioresource Technology Reports. 2019;7:1002-34. DOI: https://doi.org/10.1016/j.biteb.2019.100234
UPME, “Biocombustibles en Colombia,” Ministerio de Minas y Energía, ed., Unidad de Planeación Minero Energética. 2009; p.22. DOI: http://www.upme.gov.co/docs/biocombustibles_colombia.pdf.
Laime EM, Fernandes PD, Oliveira DC de S, Freire E de A. Possibilidades tecnológicas para a destinação da vinhaça. R. Tróp. - Ci. agr. biol. 2011;5(3):16-29. DOI: https://doi.org/10.0000/rtcab.v5i3.260.
Chowdhary P, Raj A, Bharagava RN. Environmental pollution and health hazards from distillery wastewater and treatment approaches to combat the environmental threats: a review, Chemosphere. 2018;194:229–246. DOI: http://dx.doi.org/10.1016/j.chemosphere.2017.11.163
Chowdhary P, Singh A, Chandra R, Kumar PS, Raj A, Bharagava. NR. Detection and identification of hazardous organic pollutants from distillery wastewater by GC-MS analysis and its phytotoxicity and genotoxicity evaluation by using Allium cepa and Cicer arietinum. Chemosphere. 2022;297:134123. DOI: https://doi.org/10.1016/j.chemosphere.2022.134123
Gamboa-España E, Mijangos-Cortes J, Barahona-Perez, L, Dominguez- Maldonado J, Hernandez-Zarate G, Alzategaviria, L. Vinasses: characterization and treatments. Waste Manag. Res. 2011;29:1235-50. DOI: https://doi.org/10.1177/0734242X10387313
Christofoletti CA, Escher JP, Correia JE, Marinho JFU, Fontanetti CS. Sugarcane vinasse: environmental implications of its use, Waste Manag. 2013; 33:2752–2761. DOI: http://dx.doi.org/10.1016/j.wasman.2013.09.005
Kumar S, Gopal K. Impact of distellery effluent on physiological consequences in the freshwater teleost Channa punctatus. Bull. Environ. Contam. Toxicol. 2001; 66: 617-622. DOI: https://doi.org/10.1007/s001280053
Santal, AR, Singh, NP, Saharan BS. A novel application of Paracoccus pantotrophus for the decolorization of melanoidins from distillery effluent under static conditions. J. Environ. Manag. 2016;169:78–83. DOI: https://doi.org/10.1016/j.jenvman.2015.12.016
Zhang M, Xie L, Wang Z, Lu X, Zhou Q. Using Fe(III)-coagulant-modified colloidal gas aphrons to remove bio-recalcitrant dissolved organic matter and colorants from cassava distillery wastewater. Bioresour. Technol. 2018; 268: 346–354. DOI: https://doi.org/10.1016/j.biortech.2018.08.010
Kumar V, Sharma DC. Distillery Effluent: pollution profile, eco-friendly treatment strategies: challenges and future prospects. In book: Microbial Metabolism of Xenobiotic Compounds (pp.337-357) Edition: FirstPublisher: Springer Nature Singapore; 2019; pp. 337–357. URL: https://link.springer.com/chapter/10.1007/978-981-13-7462-3_17
Oñate J, Arenas A, Ruiz A, Rivera K, Pelaez C. Evaluation of Mutagenic and Genotoxic Activity in Vinasses Subjected to Different Treatments. Water Air Soil Pollut. 2015; 226, 144. DOI: https://doi.org/10.1007/s11270-014-2250-0.
Correia JE, Christofoletti CA, Ansoar-Rodríguez Y, Guedes AT, Fontanetti CS. Comet assay and micronucleus tests on Oreochromis niloticus (Perciforme: Cichlidae) exposed to raw sugarcane vinasse and to phisicochemical treated vinasse by pH adjustment with lime (CaO). Chemosphere 2017;173:494-501. DOI: https://doi.org/10.1016/j.chemosphere.2017.01.025
Garcia CF, Souza RB, Souza CP, Christofoletti CA, Fontanetti CS. Toxicity of two effluents from agricultural activity: comparing the genotoxicity of sugar cane and orange vinasse. Ecotoxicol. Environ. Saf. 2017;142:216–221. DOI: https://doi.org/10.1016/j.ecoenv.2017.03.053
Marinho JF, Correia JE,. Marcato AC, Pedro-Escher J, Fontanetti CS. Sugar cane vinasse in water bodies: impact assessed by liver histopathology in tilapia, Ecotoxicol. Environ. Saf. 2014;110:239–245. DOI: http://dx.doi.org/10.1016/j.ecoenv.2014.09.010
Singh NP, McCoy MT, Tice RR & Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Experimental Cell Research. 1988;175(1):184-191. DOI: http://dx.doi.org/10.1016/0014-4827(88)90265-0
Ames BN, McCann J, Yamasaki E. Methods for detecting carcinogens and mutagens with the Salmonella-mammalian-microsome mutagenicity test. Mutat Res 1975; 31:347-64. DOI: https://doi.org/10.1016/0165-1161(75)90046-1
NTC3629. Norma técnica colombiana. Calidad del agua. demanda química de oxígeno (DQO). Editada por el Instituto Colombiano de Normas Técnicas y Certificación (ICONTEC). 2021. URL: https://tienda.icontec.org/catalogsearch/result/?q=NTC+3629%3A2021
NTC5167. Norma técnica colombiana. Productos para la industria agrícola. Productos orgánicos usados como abonos o fertilizantes y enmiendas o acondicionadores de suelo. Editada por el Instituto Colombiano de Normas Técnicas y Certificación (ICONTEC). 2011. URL: https://es.scribd.com/document/227264366/Norma-Tecnica-Colombiana-5167
Beltrán de Heredia AJ. Tratamiento de las aguas residuales de destilerías de vino mediante un proceso combinado delodos activos y oxidación química. Alimentacion Equipos y Tecnologia. 2005;198:55-59. URL: https://www.researchgate.net/publication/236841582
Novelo RI, Reyes RB, Borges ER and Riancho MR. Tratamiento de lixiviados por oxidación Fenton. Ingeniería e Investigación. 2010;30:80-85. URL: https://www.redalyc.org/pdf/643/64312498015.pdf
Tice RR, Agurell E, Anderson D, Burlinson B, Hartmann A, Kobayashi H, Ryu JC y Sasaki YF. Single Cell Gel/Comet Assay: Guidelines for In Vitro and In Vivo Genetic Toxicology Testing.Environ. Mol. Mutagen. 2000;35(3):206-21. DOI: https://doi.org/10.1002/(sici)1098-2280(2000)35:3%3C206::aid-m8%3E3.0.co;2-j
Maron DM, Ames BN. Revised methods for the Salmonella mutagenicity test. Mutat. Res. Mutagen. Relat. Subj. 1983;113:173–215. DOI: https://doi.org/10.1016/0165-1161(83)90010-9
Oliveira NA, Juarez B, Eurides LA, Franco D, Baungarten LA, Araujo LB. Aislamiento, viabilidad y rendimiento de células mononucleares de médula ósea de conejos. Acta Bioquímica Clínica Latinoamericana. 2011;45(1)113-118. URL: https://www.redalyc.org/pdf/535/53519965007.pdf
Gutiérrez CL . Comparación de dos técnicas para la cuantificación de la viabilidad en células mononucleares de sangre periférica evaluadas en el Hospital IV Augusto Hernández Mendoza del distrito de Ica. Tesis de maestría, Universidad Alas Peruanas. 2017.
Cooper CE,. Nitric oxide and iron proteins. Biochimica et Biophysica Acta. 1999; 1411: 290-309. DOI: https://doi.org/10.1016/S0005-2728(99)00021-3
Murphy Michael. Nitric oxide and cell death. Biochimica et Biophysica Acta. 1999;1411(2-3):401-14. DOI: https://doi.org/10.1016/S0005-2728(99)00029-8
Glösl S, Wagner KH, Draxler A, Kaniak M, Lichtenecker S, Sonnleitner A, Somoza V, Erbersdobler H, Elmadfa I. Genotoxicity and mutagenicity of melanoidins isolated from a roasted glucose-glycine model in human lymphocyte cultures, intestinal Caco-2 cells and in the Salmonella typhimurium strains TA98 and TA102 applying the AMES test. Food Chem Toxicol. 2004;42(9):1487-95. DOI: https://doi.org/10.1016/j.fct.2004.04.011
Kim Ji-S, Young-Soon Lee. Effect of reaction pH on enolization and racemization reactions of glucose and fructose on heating with amino acid enantiomers and formation of melanoidins as result of the Maillard reaction, Food Chemistry. 2008; 108(2):582-592. DOI: https://doi.org/10.1016/j.fct.2004.04.011
Kwak EJ, Lee YS, Murata M, Homma S. Effect of pH control on the intermediates and melanoidins of nonenzymatic browning reaction. Food Science and Technology. 2005;38(1):1-6. DOI: https://doi.org/10.1016/j.lwt.2003.09.011
Yadav A, Raj A, Purchase D, Ferreira LF, Saratale GD, Bharagava RN. Phytotoxicity, cytotoxicity and genotoxicity evaluation of organic and inorganic pollutants rich tannery wastewater from a Common Effluent Treatment Plant (CETP) in Unnao district, India using Vigna radiata and Allium cepa. Chemosphere. 2019;224:324-332. DOI: https://doi.org/10.1016/j.chemosphere.2019.02.124
Neuwirth C, Mosesso P, Pepe , Fiore M, Malfatti , Turteltaub K, Dekant W, Mall M. Furan carcinogenicity: DNA binding and genotoxicity of furan in rats in vivo. Molecular Nutrition & Food Research. 2012;56(9); 1363-1374. DOI: https://doi.org/10.1002/mnfr.201200226
Moser GJ, Foley J, Burnett M, Goldsworthy, TL, Maronpot R. Furan induced dose-response relationships for liver cytotoxicity, cell proliferation, and tumorigenicity (furan-induced liver tumorigenicity). Exp. Toxicol. Pathol. 2009; 61:101-111. DOI: https://doi.org/10.1016/j.etp.2008.06.006
Johansson E, Reynolds S, Anderson M, Maronpot R. Frequency of Ha-ras-1 gene mutations inversely correlated with furan dose in mouse liver tumors. Mol. Carcinog. 1997;18:199-205. DOI: https://doi.org/10.1002/(SICI)1098-2744(199704)18:4%3C199::AID-MC3%3E3.0.CO;2-9
Gramo EA y Amirtharajah A. Eliminando color causado por húmico ácidos. J. AWWA 1985, 77(3), 50–57. DOI: https://doi.org/10.1002/j.1551-8833.1985.tb05508.x
Sayato Y, Nakamuro K. Ueno H. Goto R. Identification of polycyclic aromatic hydrocarbons in mutagenic adsorbates to a copper-phthalocyanine derivative recovered from municipal river water, Mutation Research/Genetic Toxicology. 1993;300(3–4):207-213. DOI: https://doi.org/10.1016/0165-1218(93)90052-F.
White PA. The genotoxicity of priority polycyclic aromatic hydrocarbons in complex mixtures. Mutat Res. 2002;515(1-2):85-98. DOI: https://doi.org/10.1016/S1383-5718(02)00017-7
Alabi, OA. Comparative chemical analysis, mutagenicity, and genotoxicity of Petroleum refinery wastewater and its contaminated river using prokaryotic and eukaryotic assays. Protoplasma. 2023;260:89–101. DOI: https://doi.org/10.1007/s00709-022
Xu Q, Tao W, Ao Z., 2017 Antioxidant activity of vinegar melanoidins. Food Chemistry. 2007;102:841–849. DOI: https://doi.org/10.1016/j.foodchem.2006.06.013
Parnaudeau V, Condom N, Oliver R, Cazevieille P & Recous S. Vinasse organic matter quality and mineralization potential, as influenced by raw material, fermentation and concentration processes. Bioresource Technology. 2008;99:1553–1562. DOI: https://doi.org/10.1016/j.biortech.2007.04.012
Corrêa NM, Souza V, Souza TS. Genotoxic and mutagenic effects of sewage sludge on higher plants, Ecotoxicology and Environmental Safety. 2016; 24:489-496. DOI: https://doi.org/10.1016/j.ecoenv.2015.11.031
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Iván Meléndez Gélvez, Diego Alberto Salazar Moncada, Elkín Johan Granados Vega, Jennifer Carolina Soledad Maldonado, Carlos Alberto Peláez Jaramillo

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Copyright Notice and Open Access Statement
The Journal Vitae works under the Open Access license, and the published manuscripts remain available for the public, both on the Journal's website and in databases, under the Creative Commons license, "Noncommercial Attribution" and "Share alike" systems, adopted in Colombia. Hence, when the authors agree to publish in the Journal Vitae, they will not have the right to economic retributions on publications and reproductions through different diffusion media. The documents are freely available to the internet public, permitting users to read, download, copy, distribute, print, search, or link to the full texts and pass them as data to software. The only constraint on reproduction and distribution, should be to give authors control over the integrity of their work and the right to be appropriately acknowledged and cited.
Authors declare that:
-
They are the intellectual property owners and are responsible for all the information stated in the article.
-
This manuscript has not been submitted or published in other printed or digital media. They accept the responsibility for the judgments, opinions, and points of view expressed in the published article and, therefore, they exonerate Universidad de Antioquia and Journal Vitae from any process.
-
They exempt Universidad de Antioquia and Journal Vitae from settling conflicts or disputes related to the authorship of the referred article.
-
They accept the revision of the original manuscript by suitable personnel, and they bind themselves to perform the corrections appointed or suggested by the assessors.
-
Therefore, they know the editorial process and will not bind the Editorial Board of the Journal to assume any obligations regarding the volume and issue in which the article is published.
-
They transfer the rights of publication, reprinting, and distribution of the article from the moment of its approval, in print and digital format, without the right to economic rewards, and under the licensing conditions considered relevant by Journal Vitae.
-
They fully authorize Universidad de Antioquia and Journal Vitae to submit the published material to the diverse databases and indexing systems where the Journal can be found to comply with the requirements of the regulatory authorities to maintain the national classification of journals.
-
They will assume the article publication costs established for the current issue, and they will make the payment as soon as they are informed about the volume and the issue in which the final version of the article is published.
-
After the article is published, you can share digital or printed copies in a noncommercial manner. You will be able to use the paper in your institution or company for educational or research purposes, including the use in course programs.
Conflict of interest: Authors are responsible for recognizing and disclosing any financial or other benefits that could be perceived to bias their work, acknowledging all financial support and any personal connections with potential sponsors. Examples of such conflicts include receiving research funds or honoraria, serving on advisory boards, stock ownership, or employment and consulting arrangements. Authors without such connections should clearly state that they have no financial support or personal relationships that could be perceived to bias their work. All conflicts of interest should be disclosed on the author's identification page of the manuscript.










