Redes en estado de reposo: revisión y aplicaciones de un concepto en evolución
DOI:
https://doi.org/10.17533/udea.iatreia.v29n4a05Palabras clave:
análisis de componentes independientes, estado de reposoResumen
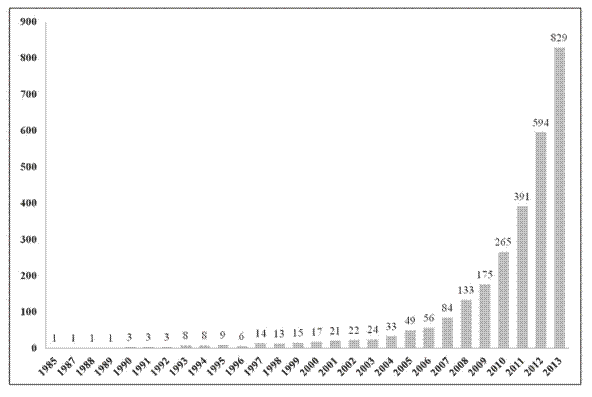
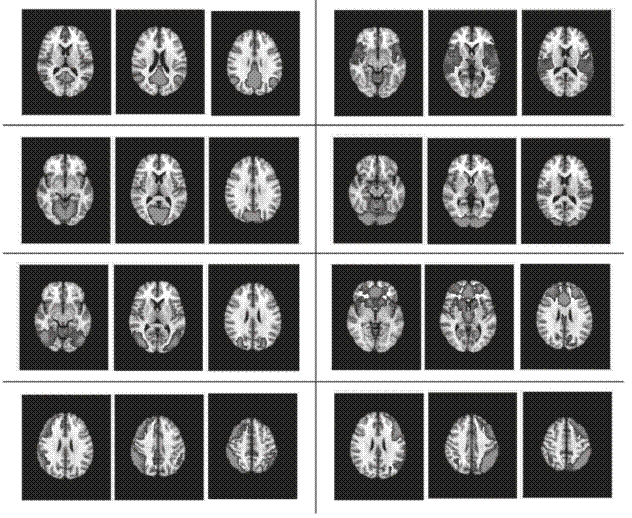
El concepto de redes en estado de reposo se refiere a fluctuaciones coherentes de la actividad cerebral, presentes en forma de redes que aparecen cuando los sujetos no están ocupados en alguna actividad o proceso cognitivo superior. Estas redes han sido identificadas en estudios de resonancia magnética funcional y reflejan un alto nivel de conectividad del cerebro humano. El concepto ha crecido en notoriedad hasta tal punto que ahora es casi rutina entre los grupos que usan resonancia magnética en sus investigaciones. Se ha estudiado la actividad de las redes en estado de reposo en el envejecimiento, el deterioro cognitivo leve y diversas enfermedades neurológicas y psiquiátricas. Además, se ha demostrado su existencia en otros primates y en ratas. En esta revisión se presenta una visión general del concepto, así como de las herramientas para la adquisición y el análisis. Igualmente se discuten algunas investigaciones relevantes sobre el tema y su impacto en diversas entidades clínicas.
Descargas
Citas
(1.) Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995 Oct;34(4):537-41.
(2.) Heeger DJ, Ress D. What does fMRI tell us about neuronal activity? Nat Rev Neurosci. 2002 Feb;3(2):142-51.
(3.) Biswal BB. Resting state fMRI: a personal history. Neuroimage. 2012 Aug;62(2):938-44. DOI 10.1016/j.neuroimage. 2012.01.090.
(4.) Biswal BB, Van Kylen J, Hyde JS. Simultaneous assessment of flow and BOLD signals in resting-state functional connectivity maps. NMR Biomed. 1997 Jun-Aug;10(4-5):165-70.
(5.) Raichle ME, Snyder AZ. A default mode of brain function: a brief istory of an evolving idea. Neuroimage. 2007 Oct;37(4):1083-90.
(6.) Wise RG, Ide K, Poulin MJ, Tracey I. Resting fluctuations in arterial carbon dioxide induce significant low frequency variations in BOLD signal. Neuroimage. 2004 Apr;21(4):1652-64.
(7.) Shmueli K, van Gelderen P, de Zwart JA, Horovitz SG, Fukunaga M, Jansma JM, et al. Low-frequency fluctuations in the cardiac rate as a source of variance in the resting-state fMRI BOLD signal. Neuroimage. 2007 Nov;38(2):306-20.
(8.) Birn RM, Diamond JB, Smith MA, Bandettini PA. Separating respiratory-variation-related fluctuations from neuronal-activity-related fluctuations in fMRI. Neuroimage. 2006 Jul;31(4):1536-48.
(9.) Birn RM, Smith MA, Jones TB, Bandettini PA. The respiration response function: the temporal dynamics of fMRI signal fluctuations related to changes in respiration. Neuroimage. 2008 Apr;40(2):644-54. DOI 10.1016/j.neuroimage.2007.11.059.
(10.) de Munck JC, Gonçalves SI, Faes TJ, Kuijer JP, Pouwels PJ, Heethaar RM, et al. A study of the brain’s resting state based on alpha band power, heart rate and fMRI. Neuroimage. 2008 Aug;42(1):112-21. DOI 10.1016/j.neuroimage.2008.04.244.
(11.) Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008 Mar;1124:1-38. DOI 10.1196/annals.1440.011.
(12.) Birn RM. The role of physiological noise in resting-state functiona connectivity. Neuroimage. 2012 Aug;62(2):864-70. DOI 10.1016/j.neuroimage.2012.01.016
(13.) Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A. 2004 Mar;101(13):4637-42.
(14.) Bluhm RL, Miller J, Lanius RA, Osuch EA, Boksman K, Neufeld RWJ, et al. Spontaneous low-frequency fluctuations in the BOLD signal in schizophrenic patients: anomalies in the default network. Schizophr Bull. 2007;33(4):1004-12. DOI 10.1093/schbul/sbm052.
(15.) Delaveau P, Salgado-Pineda P, Fossati P, Witjas T, Azulay JP, Blin O. Dopaminergic modulation of the default mode network in Parkinson’s disease. Eur Neuropsychopharmacol. 2010 Nov;20(11):784-92. DOI 10.1016/j.euroneuro.2010.07.001.
(16.) Quarantelli M, Salvatore E, Giorgio SM, Filla A, Cervo A, Russo CV, et al. Default-mode network changes in Huntington’s disease: an integrated MRI study of functional connectivity and morphometry. PLoS One. 2013 Aug;8(8):e72159. DOI 10.1371/journal.pone.0072159.
(17.) Neuner I, Werner CJ, Arrubla J, Stöcker T, Ehlen C, Wegener HP, et al. Imaging the where and when of tic generation and resting state networks in adult Tourette patients. Front Hum Neurosci. 2014 May;8:362. DOI 10.3389/fnhum.2014.00362.
(18.) Damoiseaux JS, Beckmann CF, Arigita EJ, Barkhof F, Scheltens P, Stam CJ, et al. Reduced resting-state brain activity in the “default network” in normal aging. Cereb Cortex. 2008 Aug;18(8):1856-64.
(19.) Sorg C, Riedl V, Mühlau M, Calhoun VD, Eichele T, Läer L, et al. Selective changes of resting-state networks in individuals at risk for Alzheimer’s disease. Proc Natl Acad Sci U S A. 2007 Nov;104(47):18760-5.
(20.) Glahn DC, Winkler AM, Kochunov P, Almasy L, Duggirala R, Carless MA, et al. Genetic control over the resting brain. Proc Natl Acad Sci U S A. 2010 Jan;107(3):1223-8. DOI 10.1073/pnas.0909969107.
(21.) Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van Essen DC, et al. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007 May;447(7140):83-6.
(22.) Hutchison RM, Mirsattari SM, Jones CK, Gati JS, Leung LS. Functional networks in the anesthetized rat brain revealed by independent component analysis of resting-state FMRI. J Neurophysiol. 2010 Jun;103(6):3398-406. DOI 10.1152/jn.00141.2010.
(23.) Feinberg DA, Moeller S, Smith SM, Auerbach E, Ramanna S, Gunther M, et al. Multiplexed echo planar imaging for sub-second whole brain FMRI and fast diffusion imaging. PLoS One. 2010 Dec;5(12):e15710. DOI 10.1371/journal.pone.0015710. Erratum in: PLoS One. 2011;6(9). DOI 10.1371/annotation/d9496d01-8c5d-4d24-8287-94449ada5064.
(24.) Duncan NW, Northoff G. Overview of potential procedural and participant-related confounds for neuroimaging of the resting state. J Psychiatry Neurosci. 2013 Mar;38(2):84-96. DOI 10.1503/jpn.120059.
(25.) Petridou N, Gaudes CC, Dryden IL, Francis ST, Gowland PA. Periods of rest in fMRI contain individual spontaneous events which are related to slowly fluctuating spontaneous activity. Hum Brain Mapp. 2013 Jun;34(6):1319-29. DOI 10.1002/hbm.21513.
(26.) Weissman-Fogel I, Moayedi M, Taylor KS, Pope G, Davis KD. Cognitive and default-mode resting state networks: do male and female brains “rest” differently? Hum Brain Mapp. 2010 Nov;31(11):1713-26. DOI 10.1002/hbm.20968.
(27.) Liu TT, Liau J. Caffeine increases the linearity of the visual BOLD response. Neuroimage. 2010 Feb;49(3):2311-7. DOI 10.1016/j.neuroimage.2009.10.040.
(28.) Rack-Gomer AL, Liu TT. Caffeine increases the temporal variability of resting-state BOLD connectivity in the motor cortex. Neuroimage. 2012 Feb;59(3):2994-3002. DOI 10.1016/j.neuroimage.2011.10.001.
(29.) Song XW, Dong ZY, Long XY, Li SF, Zuo XN, Zhu CZ, et al. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One. 2011;6(9):e25031. DOI 10.1371/journal.pone.0025031.
(30.) Chao-Gan Y, Yu-Feng Z. DPARSF: A MATLAB Toolbox for “Pipeline” Data Analysis of Resting-State fMRI. Front Syst Neurosci. 2010 May;4:13. DOI 10.3389/fnsys. 2010.00013.
(31.) Biswal BB, Ulmer JL. Blind source separation of multiple signal sources of fMRI data sets using independent component analysis. J Comput Assist Tomogr. 1999 Mar-Apr;23(2):265-71.
(32.) Beckmann CF, Smith SM. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans Med Imaging. 2004 Feb;23(2):137-52.
(33.) Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci. 2005 May;360(1457):1001-13.
(34.) Kiviniemi V, Kantola JH, Jauhiainen J, Hyvärinen A, Tervonen O. Independent component analysis of nondeterministic fMRI signal sources. Neuroimage. 2003 Jun;19(2 Pt 1):253-60.
(35.) Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, et al. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci U S A. 2009 Aug;106(31):13040-5. DOI 10.1073/pnas.0905267106.
(36.) Zuo XN, Kelly C, Adelstein JS, Klein DF, Castellanos FX, Milham MP. Reliable intrinsic connectivity networks: test-retest evaluation using ICA and dual regression approach. Neuroimage. 2010 Feb;49(3):2163-77. DOI 10.1016/j.neuroimage.2009.10.080.
(37.) Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001 Jan;98(2):676-82.
(38.) Fransson P. Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Hum Brain Mapp. 2005 Sep;26(1):15-29.
(39.) Snyder AZ, Raichle ME. A brief history of the resting state: the Washington University perspective. Neuroimage. 2012 Aug;62(2):902-10. DOI 10.1016/j.neuroimage.2012.01.044.
(40.) Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. Neuroimage. 2007 Oct;37(4):1083-90.
(41.) Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003 Jan;100(1):253-8.
(42.) Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005 Jul;102(27):9673-8.
(43.) Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006 Mar;129(Pt 3):564-83.
(44.) Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VJ, et al. Mapping the structural core of human cerebral cortex. PLoS Biol. 2008 Jul;6(7):e159. DOI 10.1371/journal.pbio.0060159.
(45.) Vogt BA, Finch DM, Olson CR. Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cereb Cortex. 1992 Nov-Dec;2(6):435-43.
(46.) Leech R, Sharp DJ. The role of the posterior cingulate cortex in cognition and disease. Brain. 2014 Jan;137(Pt 1):12-32. DOI 10.1093/brain/awt162.
(47.) Vogt BA, Laureys S. Posterior cingulate, precuneal and retrosplenial cortices: cytology and components of the neural network correlates of consciousness. Prog Brain Res. 2005;150:205-17.
(48.) Neuner I, Arrubla J, Werner CJ, Hitz K, Boers F, Kawohl W, et al. The default mode network and EEG regional spectral power: a simultaneous fMRI-EEG study. PLoS One. 2014 Feb;9(2):e88214. DOI 10.1371/journal.pone.0088214.
(49.) Nunez PL, Silberstein RB. On the relationship of synaptic activity to macroscopic measurements: does co-registration of EEG with fMRI make sense? Brain Topogr. 2000 Winter;13(2):79-96.
(50.) Laufs H, Krakow K, Sterzer P, Eger E, Beyerle A, Salek-Haddadi A, et al. Electroencephalographic signatures of attentional and cognitive default modes in spontaneous brain activity fluctuations at rest. Proc Natl Acad Sci U S A. 2003 Sep;100(19):11053-8.
(51.) Shah NJ, Oros-Peusquens AM, Arrubla J, Zhang K, Warbrick T, Mauler J, et al. Advances in multimodal neuroimaging: hybrid MR-PET and MR-PET-EEG at 3 T and 9.4 T. J Magn Reson. 2013 Apr;229:101-15. DOI 10.1016/j.jmr.2012.11.027.
(52.) Fox PT, Raichle ME. Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects. Proc Natl Acad Sci U S A. 1986 Feb;83(4):1140-4.
(53.) Malonek D, Dirnagl U, Lindauer U, Yamada K, Kanno I, Grinvald A. Vascular imprints of neuronal activity: relationships between the dynamics of cortical blood flow, oxygenation, and volume changes following sensory stimulation. Proc Natl Acad Sci U S A. 1997 Dec;94(26):14826-31.
(54.) Malonek D, Grinvald A. Interactions between electrical activity and cortical microcirculation revealed by imaging spectroscopy: implications for functional brain mapping. Science. 1996;272(5261):551-4.
(55.) Jann K, Kottlow M, Dierks T, Boesch C, Koenig T. Topographic electrophysiological signatures of FMRI Resting State Networks. PLoS One. 2010 Sep;5(9):e12945. DOI 10.1371/journal.pone.0012945.
(56.) Duncan NW, Wiebking C, Muñoz-Torres Z, Northoff G. How to investigate neuro-biochemical relationships on a regional level in humans? Methodological considerations for combining functional with biochemical imaging. J Neurosci Methods. 2014 Jan;221:183-8.
(57.) Arrubla J, Tse DH, Amkreutz C, Neuner I, Shah NJ. GABA concentration in posterior cingulate cortex predicts putamen response during resting state fMRI. PLoS One. 2014 Sep;9(9):e106609. DOI 10.1371/journal.pone.0106609.
(58.) Enzi B, Duncan NW, Kaufmann J, Tempelmann C, Wiebking C, Northoff G. Glutamate modulates resting state activity in the perigenual anterior cingulate cortex – a combined fMRI-MRS study. Neuroscience. 2012 Dec;227:102-9. DOI 10.1016/j.neuroscience.2012.09.039.
(59.) Northoff G, Walter M, Schulte RF, Beck J, Dydak U, Henning A, et al. GABA concentrations in the human anterior cingulate cortex predict negative BOLD responses in fMRI. Nat Neurosci. 2007 Dec;10(12):1515-7.
(60.) Kapogiannis D, Reiter DA, Willette AA, Mattson MP. Posteromedial cortex glutamate and GABA predict intrinsic functional connectivity of the default mode network. Neuroimage. 2013 Jan;64:112-9. DOI 10.1016/j.neuroimage.2012.09.029.
(61.) Strittmatter WJ, Weisgraber KH, Huang DY, Dong LM, Salvesen GS, Pericak-Vance M, et al. Binding of human apolipoprotein E to synthetic amyloid beta peptide: isoform-specific effects and implications for late-onset Alzheimer disease. Proc Natl Acad Sci U S A. 1993 Sep;90(17):8098-102.
(62.) Filippini N, MacIntosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM, et al. Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proc Natl Acad Sci U S A. 2009 Apr;106(17):7209-14. DOI 10.1073/pnas.0811879106.
(63.) Werner CJ, Dogan I, Saß C, Mirzazade S, Schiefer J, Shah NJ, et al. Altered resting-state connectivity in Huntington’s disease. Hum Brain Mapp. 2014 Jun;35(6):2582-93. DOI 10.1002/hbm.22351.
(64.) Harrison BJ, Yücel M, Pujol J, Pantelis C. Task-induced deactivation of midline cortical regions in schizophrenia assessed with fMRI. Schizophr Res. 2007 Mar;91(1-3):82-6.
(65.) Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci U S A. 2009 Jan;106(4):1279-84. DOI 10.1073/pnas.0809141106. Erratum in: Proc Natl Acad Sci U S A. 2009 Mar;106(11):4572.
(66.) Zhou Y, Liang M, Tian L, Wang K, Hao Y, Liu H, et al. Functional disintegration in paranoid schizophrenia using resting-state fMRI. Schizophr Res. 2007 Dec;97(1-3):194-205.
(67.) Garrity AG, Pearlson GD, McKiernan K, Lloyd D, Kiehl KA, Calhoun VD. Aberrant “default mode” functional connectivity in schizophrenia. Am J Psychiatry. 2007 Mar;164(3):450-7. Erratum in: Am J Psychiatry. 2007 Jul;164(7):1123.
(68.) Jafri MJ, Pearlson GD, Stevens M, Calhoun VD. A method for functional network connectivity among spatially independent resting-state components in schizophrenia. Neuroimage. 2008 Feb;39(4):1666-81.
(69.) Liu H, Buckner RL, Talukdar T, Tanaka N, Madsen JR, Stufflebeam SM. Task-free presurgical mapping using functional magnetic resonance imaging intrinsic activity. J Neurosurg. 2009 Oct;111(4):746-54. DOI 10.3171/2008.10.JNS08846.
(70.) Zhang D, Johnston JM, Fox MD, Leuthardt EC, Grubb RL, Chicoine MR, et al. Preoperative sensorimotor mapping in brain tumor patients using spontaneous fluctuations in neuronal activity imaged with functional magnetic resonance imaging: initial experience. Neurosurgery. 2009 Dec;65(6 Suppl):226-36. DOI 10.1227/01.NEU.0000350868.95634.CA.
(71.) Boly M, Phillips C, Tshibanda L, Vanhaudenhuyse A, Schabus M, Dang-Vu TT, et al. Intrinsic brain activity in altered states of consciousness: how conscious is the default mode of brain function? Ann N Y Acad Sci. 2008;1129:119-29. DOI 10.1196/annals.1417.015.
(72.) Cauda F, Micon BM, Sacco K, Duca S, D’Agata F, Geminiani G, et al. Disrupted intrinsic functional connectivity in the vegetative state. J Neurol Neurosurg Psychiatry. 2009 Apr;80(4):429-31. DOI 10.1136/jnnp.2007.142349.
(73.) Vanhaudenhuyse A, Noirhomme Q, Tshibanda LJ, Bruno MA, Boveroux P, Schnakers C, et al. Default network connectivity reflects the level of consciousness in noncommunicative brain-damaged patients. Brain. 2010 Jan;133(Pt 1):161-71. DOI 10.1093/brain/awp313.
Publicado
Cómo citar
Número
Sección
Licencia
Derechos de autor 2016 Iatreia

Esta obra está bajo una licencia internacional Creative Commons Atribución-CompartirIgual 4.0.
Los artículos publicados en la revista están disponibles para ser utilizados bajo la licencia Creative Commons, específicamente son de Reconocimiento-NoComercial-CompartirIgual 4.0 Internacional.
Los trabajos enviados deben ser inéditos y suministrados exclusivamente a la Revista; se exige al autor que envía sus contribuciones presentar los formatos: presentación de artículo y responsabilidad de autoría completamente diligenciados.