In vitro antimicrobial activity of Caesalpinia coriaria (Jacq.) Willd extracts on Streptococcus pyogenes and Candida albicans
DOI:
https://doi.org/10.17533/udea.vitae.v28n2a345381Keywords:
Caesalpinia coriaria extract, antimicrobial activity, Minimum Inhibitory Concentration, Streptococcus pyogenes, Candida albicansAbstract
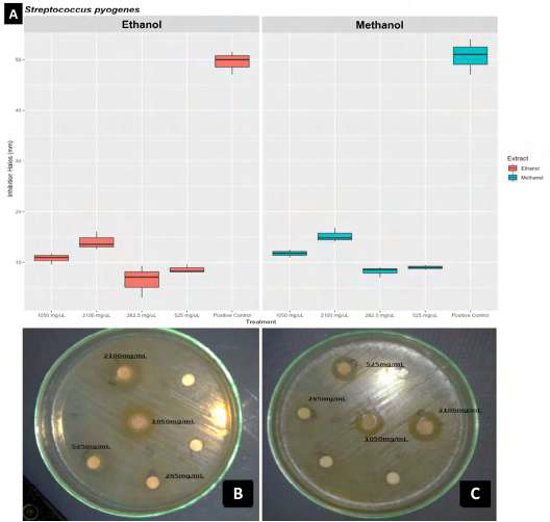
Background: “Dividivi” Caesalpinia coriaria (Jacq.) Willd fruits are traditionally used by the Wayuú community in La Guajira (Colombia) to treat oral and skin cavity diseases caused by bacteria and fungi. Streptococcus pyogenes is a gram-positive cocci of group A (beta-hemolytic) that is the cause of pharyngeal disease, scarlet fever, cellulitis, erysipelas, or toxic shock-like syndrome. Alternatively, Candida albicans is a yeast-like fungus that is a normal flora of the digestive tract, vagina, or skin folds; it has been known to be the root cause of opportunistic diseases such as diaper rash, oral and esophagus thrush, or vulvovaginitis. Objective: This study evaluated the antimicrobial activity of methanolic and ethanolic extracts of C. coriaria (Jacq.) Willd dry fruits on S. pyogenes ATCC 12384 and C. albicans ATTC 14053. Method: C. coriaria extracts were obtained from the Soxhlet method using two solvents (methanol and ethanol 98%) prepared from pulverized fruits. A phytochemical test and an antimicrobial activity assay were performed using the obtained extracts and tested using S. pyogenes ATCC 12384 and C. albicans ATTC 14053 strains. Results: A phytochemical profile was performed, examining the presence of bioactive metabolites (tannins, alkaloids, glycosides, saponins, and anthraquinones) from each extract. Antimicrobial susceptibility tests showed that the ethanolic extract inhibited S. pyogenes ATCC 12384, causing inhibition halos of 14.1 ± 0.1 mm and a Minimum Inhibitory Concentration (MIC) of 172 mg/ml, and C. albicans test shows inhibition halos of 16.1 ± 0.2 mm and MIC of 212 mg/ml. Additionally, the methanolic extract inhibited S. pyogenes with inhibition halos of 15.2 ± 0.2 mm and MIC of 152 mg/ml; no inhibitory effect was observed on C. albicans. Conclusion: This study revealed that C. coriaria has an antimicrobial effect on the tested species opening the field of its possible use as a therapeutic agent.
Downloads
References
Sen T, Samanta SK. Medicinal plants, human health and biodiversity: a broad review. Advances in biochemical engineering/biotechnology. 2015; 147: 59-110. https://doi.org/10.1007/10_2014_273
Anand U, Jacobo-Herrera N, Altemimi A, Lakhssassi N. A Comprehensive Review on Medicinal Plants as Antimicrobial Therapeutics: Potential Avenues of Biocompatible Drug Discovery. Metabolites. 2019; 9(11): 258. https://doi.org/10.3390/metabo9110258
AlSheikh HMA, Sultan I, Kumar V, Rather IA, Al-Sheikh H, Tasleem Jan A, Haq QMR. Plant-Based Phytochemicals as Possible Alternative to Antibiotics in Combating Bacterial Drug Resistance. Antibiotics (Basel, Switzerland). 2020; 9(8): 480. https://doi.org/10.3390/antibiotics9080480
Gupta C, Prakash D. Phytonutrients as therapeutic agents. Journal of complementary & integrative medicine. 2014; 11(3): 151–169. https://doi.org/10.1515/jcim-2013-0021
Zanin JL, de Carvalho BA, Martineli PS, dos Santos MH, Lago JH, Sartorelli P, Viegas C Jr, Soares MG. The genus Caesalpinia L. (Caesalpiniaceae): phytochemical and pharmacological characteristics. Molecules (Basel, Switzerland). 2012; 17(7):7887-7902. https://doi.org/10.3390/molecules17077887
Arulmozhi P, Vijayakumar S, Kumar T. Phytochemical analysis and antimicrobial activity of some medicinal plants against selected pathogenic microorganisms. Microbial pathogenesis. 2018; 123: 219–226. https://doi.org/10.1016/j.micpath.2018.07.009
Pájaro González Y, Méndez Cuadro D, Fernández Daza E, et al. Inhibitory activity of the protein carbonylation and hepatoprotective effect of the ethanol-soluble extract of Caesalpinia coriaria Jacq. Orient Pharm Exp Med, 2016; 16: 225–232. https://doi.org/10.1007/s13596-016-0228-8
Sánchez-Carranza JN, Alvarez L, Marquina-Bahena S, Salas-Vidal E, Cuevas V, Jiménez EW, Veloz G RA, Carraz M, González-Maya L. Phenolic Compounds Isolated from Caesalpinia coriaria Induce S and G2/M Phase Cell Cycle Arrest Differentially and Trigger Cell Death by Interfering with Microtubule Dynamics in Cancer Cell Lines. Molecules (Basel, Switzerland),. 2017; 22(4): 666. https://doi.org/10.3390/molecules22040666
Manuel-Pablo A, Elghandour MMY, Olivares-Pérez J, et al. Productive performance, rumen fermentation and carcass yield of goats supplemented with cascalote fruit (Caesalpinia coriaria J. Wild.). Agroforest Syst. 2020; 94: 1381–1391. https://doi.org/10.1007/s10457-018-0312-9
García-Hernández C, Rojo-Rubio R, Olmedo-Juárez A, Zamilpa A, Mendoza de Gives P, Antonio-Romo IA, Aguilar-Marcelino L, Arece-García J, Tapia-Maruri D, González-Cortazar M. Galloyl derivatives from Caesalpinia coriaria exhibit in vitro ovicidal activity against cattle gastrointestinal parasitic nematodes. Experimental parasitology. 2019; 200, 16-23. https://doi.org/10.1016/j.exppara.2019.03.012
Gallego MG, Rodríguez T, Rodríguez I, Almajano MP. Analytical Characterization of Polyphenols from Tara and Caesalpinia decapetala as Stabilizers of O/W Emulsions. Journal of food science. 2016; 81(11): C2676–C2685. https://doi.org/10.1111/1750-3841.13502
Walker MJ, Barnett TC, McArthur JD, Cole JN, Gillen CM, Henningham A, Sriprakash KS, Sanderson-Smith ML, Nizet V. Disease manifestations and pathogenic mechanisms of Group A Streptococcus. Clinical microbiology reviews. 2014; 27(2): 264–301. https://doi.org/10.1128/CMR.00101-13
Mayer FL, Wilson D, Hube B. Candida albicans pathogenicity mechanisms. Virulence. 2013; 4(2): 119-128. https://doi.org/10.4161/viru.22913
Kiraz N, Oz Y. Species distribution and in vitro antifungal susceptibility of clinical Candida isolates from a university hospital in Turkey over a 5-year period. Medical mycology. 2011; 49(2): 126–131. https://doi.org/10.3109/13693786.2010.503195
Rosado JR, Moreno MI. Farmacopea guajira: el uso de las plantas medicinales xerofíticas por la etnia wayuu. Revista CENIC. Ciencias Biológicas. 2010; 41:1-10.
Lennon RP, Lopez K, Socha J, Montealegre F, Chandler JW, Sweet NN, Hawley LA, Smith DK, Sanchack KE. Health Characteristics of the Wayuu Indigenous People. Military medicine. 2019; 184(7-8): e230–e235. https://doi.org/10.1093/milmed/usz021
De Jesús-Martínez X, Olmedo-Juárez A, Olivares-Pérez J, Zamilpa A, Mendoza de Gives P, López-Arellano ME, Rojas-Hernández S, Villa-Mancera A, Camacho-Díaz LM, Cipriano-Salazar M. In Vitro Anthelmintic Activity of Methanolic Extract from Caesalpinia coriaria J. Willd Fruits against Haemonchus contortus Eggs and Infective Larvae. BioMed research international. 2018, 7375693. https://doi.org/10.1155/2018/7375693
Valencia, E. F., Mac Donald, D., Cuyos, M., & Dueñas, R. Extracción, identificación y evaluación de saponinas en agaricus bisporus. 2017, 5: https://doi.org/10.31381/biotempo.v5i0.889
Opinde, H. R., Nyamache, A. K., & Gatheri, G. W. Antimicrobial activity, qualitative phytochemical composition of crude extracts from medicinal plants against selected enteric bacterial pathogens, Candida albicans. 2018. Bioteknologi Biotechnological Studies, 15(1), 1-12. https://doi.org/10.13057/biotek/c150101
Torres Chati, J. Evaluación de la actividad antimicrobiana de extractos de Luma chequen (Molina) A. Gray" Arrayán" frente a patógenos aislados de hemocultivos del Hospital Nacional Guillermo Almenara Irigoyen, 2014. Lima-Perú. Available from: https://cybertesis.unmsm.edu.pe/handle/20.500.12672/3605 Access: march 25th
Cruz-Carrillo, A., Rodríguez, N., & Rodríguez, C. E. In vitro evaluation of the antibacterial effect of Bidens pilosa, Lantana camara, Schinus molle and Silybum marianum. Revista UDCA Actualidad & Divulgación Científica. 2010. 13(2): 117-124.
Orozco Hayek Marcela. Eleccion de las condiciones más adecuadas para la obtencion de extractos de plantas superiores con actividad sobre una cepa de Staphylococcus aureus resistente.tesis maestria. 2004. Universidad Autonoma de Nuevo Leon. Facultad de medicina. Access on: http://eprints.uanl.mx/6668/1/1080123958.PDF
Xavier, L.; Freire, M.S.; Vidal-Tato, I.; González-Álvarez, J. Application of aqueous two phase systems based on polyethylene glycol and sodium citrate for the recovery of phenolic compounds from Eucalyptus wood. Maderas. Ciencia y Tecnología 2015. 17(2):345-354. http://dx.doi.org/10.4067/S0718-221X2015005000032
Soto-García, Marcela, & Rosales-Castro, Martha. Efecto del solvente y de la relación masa/solvente, sobre la extracción de compuestos fenólicos y la capacidad antioxidante de extractos de corteza de Pinus durangensis y Quercus sideroxyla. Maderas. Ciencia y tecnología. 2016. 18(4): 701-714 https://dx.doi.org/10.4067/S0718-221X2016005000061
López C. R., Sarmiento C., Espitia L., Barrero A.M., Consuegra C., Gallego C., B. 2016. Divididi: Ichi Caesalpinia coriaria. En: López C. R., Sarmiento C., Espitia L., Barrero A.M., Consuegra C., Gallego C., B. 2016. 100 plantas del Caribe colombiano. Usar para conservar: aprendiendo de los habitantes del bosque seco. Fondo Patrimonio Natural, Bogotá D.C. Colombia. 75-76
Gina M. Rodríguez M., Karina Banda-R., Sandra Paola Reyes B. y Ana Cristina Estupiñán González. Lista comentada de las plantas vasculares de bosques secos prioritarios para la conservación en los departamentos de Atlántico y Bolívar (Caribe colombiano). Biota Colombiana, Vol 13 No 2 Julio-Diciemnbre, Especial Bosque Seco en Colombia.
Mora-Santacruz, Antonio., Roman-Mirando, Maria., González-Cuevas, Gerardo., Barrientos-Ramirez, Lucia. Chemical composition of cascalote Caesalpinia coriaria (Jacq.) Willd. and diversity of uses in the rural areas of dry tropics. Revista de Investigación y Desarrollo. 2018, 4(12): 24-28
Olmedo-Juárez A, Briones-Robles TI, Zaragoza-Bastida A, Zamilpa A, Ojeda-Ramírez D, Mendoza de Gives P, Olivares-Pérez J, Rivero-Perez N. Antibacterial activity of compounds isolated from Caesalpinia coriaria (Jacq) Willd against important bacteria in public health. Microbial pathogenesis. 2019; 136: 103660. https://doi.org/10.1016/j.micpath.2019.103660
Mohana DC, Satish S, Raveesha KA. Antibacterial Evaluation of Some Plant Extracts Against Some Human Pathogenic Bacteria. Advances in biological research, 2008; 2(3-4), 49-55.
Anandhi D, Srinivasan PT, Revathi K, Revathy EK. Antibacterial Activity of Caesalpinia coriaria. Biosciences Biotechnology Research Asia. 2011; 8:759-764.
Rojas J.; Velasco J.; Buitrago A., Mender T.; Rojas J. (2016). Evaluación de la actividad antimicrobiana de plantas medicinales seleccionadas del Jardín Botánico del Orinoco, municipio Heres, Estado Bolívar. Rev Fac Farm. 2016; 58(1): 2-10.
Kumar P, Bhatt RP, Singh L, Sati OP, Khan A, Ahmad A. Antimicrobial activities of essential oil and methanol extract of Coriaria nepalensis. Natural product research. 2011; 25(11): 1074–1081. https://doi.org/10.1080/14786419.2010.529545
Sampaio FC, Pereira Mdo S, Dias CS, Costa VC, Conde NC, Buzalaf MA. In vitro antimicrobial activity of Caesalpinia ferrea Martius fruits against oral pathogens. Journal of ethnopharmacology. 2009: 124(2), 289–294. https://doi.org/10.1016/j.jep.2009.04.034
Thippeswamy S, Mohana DC, Manjunath K. (2012). Screening of in vitro antifungal activity of some indian medicinal plants against Candida albicans and Cryptococcus neoformans. International Journal of Current Research. 2012; 4(3): 37-42.
Bhat PB, Hegde S, Upadhya V, Hegde GR, Habbu PV, Mulgund GS. Evaluation of wound healing property of Caesalpinia mimosoides Lam. Journal of ethnopharmacology, 2016; 193: 712–724. https://doi.org/10.1016/j.jep.2016.10.009
Soares, M. R., Corrêa, R. O., Stroppa, P. H. F., Marques, F. C., Andrade, G. F., Corrêa, C. C., & Raposo, N. R. Biosynthesis of silver nanoparticles using Caesalpinia ferrea (Tul.) Martius extract: physicochemical characterization, antifungal activity and cytotoxicity. 2018. PeerJ, 6, e4361. https://doi.org/10.7717/peerj.4361
Sharma V, Lobo R, Singh G, Chanana V, Kalsi V ,Suttee A. Antimicrobial Evaluation of Caesalpinia decapetala, 2017. IJPPR, Volume 9 (12): 1421-1424. rch 2017; 9(12); 1421-1424. https://doi.org/10.25258/phyto.v9i11.11185
Glauber P. Oliveira; Tatiane P. Souza; Sheila K. Caetano; Kaliny S. Farias; Gisely N. Venancio; Maria F. C. L. Bandeira1; Nikeila C. O. Conde. Atividade antimicrobiana in vitro de extratos da casca do caule e da vagem de Libidibia ferrea L. frente a microrganismos da cavidade bucal Revista Fitos, Rio de Janeiro. 2013. Vol. 8(2): 73-160.
Published
How to Cite
Issue
Section
License
Copyright (c) 2021 Silvio Alejandro Lopez-Pazos, Leanis Pitre-Ruiz, Deycis Galván-Ayala, Kelly Johanna Ávila Mendez, Omar Castro-Uriana

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Copyright Notice and Open Access Statement
The Journal Vitae works under the Open Access license, and the published manuscripts remain available for the public, both on the Journal's website and in databases, under the Creative Commons license, "Noncommercial Attribution" and "Share alike" systems, adopted in Colombia. Hence, when the authors agree to publish in the Journal Vitae, they will not have the right to economic retributions on publications and reproductions through different diffusion media. The documents are freely available to the internet public, permitting users to read, download, copy, distribute, print, search, or link to the full texts and pass them as data to software. The only constraint on reproduction and distribution, should be to give authors control over the integrity of their work and the right to be appropriately acknowledged and cited.
Authors declare that:
-
They are the intellectual property owners and are responsible for all the information stated in the article.
-
This manuscript has not been submitted or published in other printed or digital media. They accept the responsibility for the judgments, opinions, and points of view expressed in the published article and, therefore, they exonerate Universidad de Antioquia and Journal Vitae from any process.
-
They exempt Universidad de Antioquia and Journal Vitae from settling conflicts or disputes related to the authorship of the referred article.
-
They accept the revision of the original manuscript by suitable personnel, and they bind themselves to perform the corrections appointed or suggested by the assessors.
-
Therefore, they know the editorial process and will not bind the Editorial Board of the Journal to assume any obligations regarding the volume and issue in which the article is published.
-
They transfer the rights of publication, reprinting, and distribution of the article from the moment of its approval, in print and digital format, without the right to economic rewards, and under the licensing conditions considered relevant by Journal Vitae.
-
They fully authorize Universidad de Antioquia and Journal Vitae to submit the published material to the diverse databases and indexing systems where the Journal can be found to comply with the requirements of the regulatory authorities to maintain the national classification of journals.
-
They will assume the article publication costs established for the current issue, and they will make the payment as soon as they are informed about the volume and the issue in which the final version of the article is published.
-
After the article is published, you can share digital or printed copies in a noncommercial manner. You will be able to use the paper in your institution or company for educational or research purposes, including the use in course programs.
Conflict of interest: Authors are responsible for recognizing and disclosing any financial or other benefits that could be perceived to bias their work, acknowledging all financial support and any personal connections with potential sponsors. Examples of such conflicts include receiving research funds or honoraria, serving on advisory boards, stock ownership, or employment and consulting arrangements. Authors without such connections should clearly state that they have no financial support or personal relationships that could be perceived to bias their work. All conflicts of interest should be disclosed on the author's identification page of the manuscript.









