In Vitro evaluation of the antioxidant and hypoglycemic activities of leaves extracts from Ambrosia arborescens, Buddleja incana, Aloysia citrodora, and Prunus serotina.
DOI:
https://doi.org/10.17533/udea.vitae.v32n2a360310Keywords:
Diabetes mellitus, Phytochemical screening, Metabolites, Antioxidants, Hypoglycemic, α-amylaseAbstract
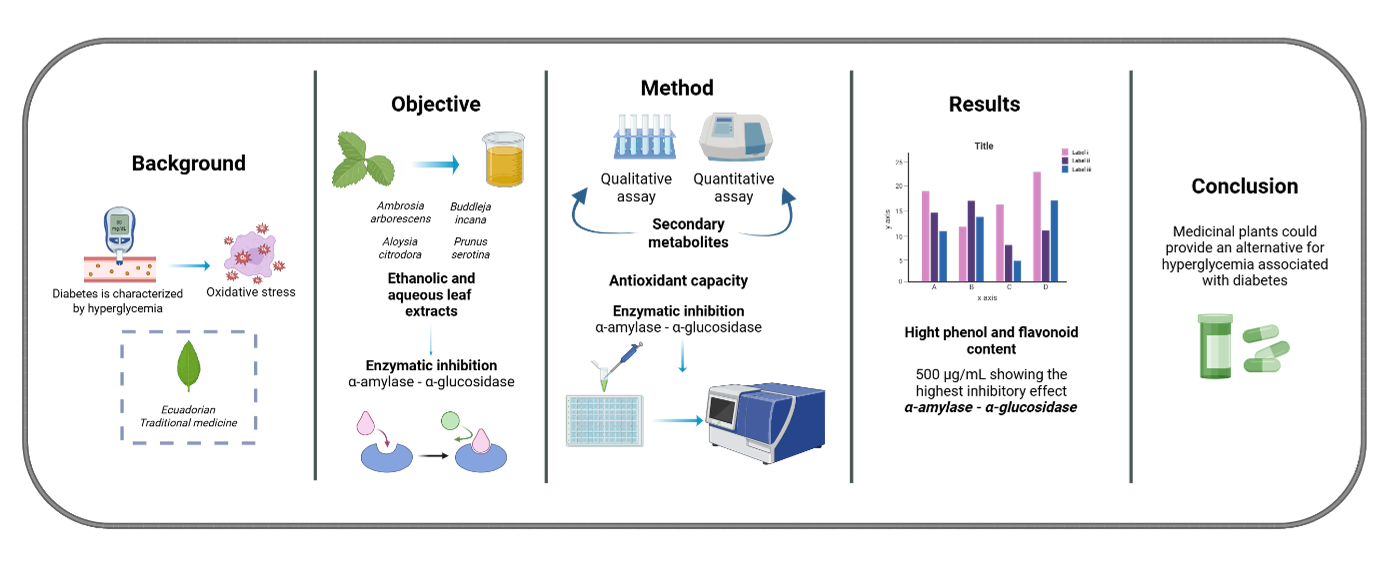
Background: Diabetes mellitus is a chronic disease affecting many people in the world. The main symptom of diabetes is high blood glucose levels (hyperglycemia), which triggers an imbalance in the body, producing secondary pathologies associated with oxidative stress generated by this metabolic disorder. Objective: This research evaluated the antioxidant and hypoglycemic capacity of Ambrosia arborescens, Buddleja incana, Aloysia citrodora, and Prunus serotina ethanolic and aqueous leaf extracts. Methods: The phytochemical profile of each plant species was characterized through qualitative tests to determine the presence or absence of metabolites such as alkaloids, phenols, triterpenes, and flavonoids. Quantitative determinations of total phenols and flavonoid content were also conducted. The free radical scavenging assay with 2,2-diphenyl-1picrylhydrazil (DPPH) evaluated the antioxidant capacity. The hypoglycemic capacity was performed by quantifying the inhibition capacity of α-amylase and α-glucosidase enzymes. Results: All extracts showed a high concentration of phenols and flavonoids. Likewise, all extracts exhibited enzymatic inhibition at different concentrations, with 500 µg/mL showing the highest inhibitory effect. Additionally, the ethanolic extract of A. arborescens demonstrated the most excellent hypoglycemic capacity among all the extracts analyzed. Conclusion: The results of this study can serve as a basis for future research focused on utilizing medicinal plants to develop pharmaceutical formulations as an alternative treatment for hyperglycemia associated with diabetes.
Downloads
References
Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843. DOI: https://doi.org/10.1016/j.diabres.2019.107843
Bhatti JS, Bhatti GK, Reddy PH. Mitochondrial dysfunction and oxidative stress in metabolic disorders — A step towards mitochondria based therapeutic strategies. Biochim Biophys Acta Mol Basis Dis. 2017;1863(5):1066–1077. DOI: https://doi.org/10.1016/j.bbadis.2016.11.010.
Darenskaya MA, Kolesnikova LI, Kolesnikov SI. Oxidative stress: Pathogenetic role in diabetes mellitus and its complications and therapeutic approaches to correction. Bull Exp Biol Med. 2021;171(2):179-89. DOI: https://doi.org/10.1007/s10517-021-05191-7 .
Retnakaran R, Pu J, Emery A, Harris SB, Reichert SM, Gerstein HC, et al. Determinants of sustained stabilization of beta-cell function following short-term insulin therapy in type 2 diabetes. Nat Commun. 2023;14:4514. DOI: https://doi.org/10.1038/s41467-023-40287-w
Borse SP, Chhipa AS, Sharma V, Singh DP, Nivsarkar M. Management of type 2 diabetes: Current strategies, unfocussed aspects, challenges, and alternatives. Med Princ Pract. 2021;30(2):109-21. DOI: https://doi.org/10.1159/000511002
Masenga SK, Kabwe LS, Chakulya M, Kirabo A. Mechanisms of oxidative stress in metabolic syndrome. Int J Mol Sci. 2023;24(9):7898. DOI: https://doi.org/10.3390/ijms24097898
Yuan H, Ma Q, Ye L, Piao G. The traditional medicine and modern medicine from natural products. Molecules. 2016;21(5):559. DOI: https://doi.org/10.3390/molecules21050559
Lim T, Davis EO, Crudge B, Roth V, Glikman JA. Traditional Khmer medicine and its role in wildlife use in modern-day Cambodia. J Ethnobiol Ethnomed. 2022;18(1):61. DOI: https://doi.org/10.1186/s13002-022-00553-5
Li Y, Kong D, Fu Y, Sussman MR, Wu H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol Biochem. 2020;148:80-9. DOI: https://doi.org/10.1016/j.plaphy.2020.01.006
Zhaogao L, Yaxuan W, Mengwei X, Haiyu L, Lin L, Delin X. Molecular mechanism overview of metabolite biosynthesis in medicinal plants. Plant Physiol Biochem. 2023;204:108125. DOI: https://doi.org/10.1016/j.plaphy.2023.108125
Yedjou CG, Grigsby J, Mbemi A, Nelson D, Mildort B, Latinwo L, et al. The management of diabetes mellitus using medicinal plants and vitamins. Int J Mol Sci. 2023;24(10):9085. DOI: https://doi.org/10.3390/ijms24109085
De la Torre L, Navarrete H, Muriel M, Macía MJ, Balslev H, Editores. Enciclopedia de las Plantas Útiles del Ecuador, 1ª ed. Herbario QCA & Herbario AAU. Quito & Aarhus: Pontificia Universidad Católica del Ecuador & Universidad de Aarhus; 2008. 949 p.
Salles BCC, da Silva MA, Taniguthi L, Ferreira JN, da Rocha CQ, Vilegas W, et al. Passiflora edulis Leaf Extract: Evidence of Antidiabetic and Antiplatelet Effects in Rats. Biol Pharm Bull. 2020;43(1):169–74. DOI: https://doi.org/10.1248/bpb.b18-00952. .
Urrego N, Sepúlveda P, Aragón M, Ramos FA, Costa GM, Ospina LF, et al. Flavonoids and saponins from Passiflora edulis f. edulis leaves (purple passion fruit) and its potential anti-inflammatory activity. J Pharm Pharmacol. 2021;73(11):1530–8. DOI: doi: https://doi.org/10.1093/jpp/rgab117.
Harborne JB. General Procedures and Measurement of Total Phenolics. In: Methods in Plant Biochemistry. Vol 1. 1st ed. London: Academic Press; 1989. p. 1–28.
Baek SH, Cao L, Jeong SJ, Kim HR, Nam TJ, Lee SG. The comparison of total phenolics, total antioxidant, and anti-tyrosinase activities of Korean Sargassum species. J Food Qual. 2021;2021:6640789. DOI: https://doi.org/10.1155/2021/6640789
N’guessan B, Asiamah A, Arthur N, Frimpong-Manso S, Amoateng P, Amponsah S, et al. Ethanolic extract of Nymphaea lotus L. (Nymphaeaceae) leaves exhibits in vitro antioxidant, in vivo anti-inflammatory and cytotoxic activities on Jurkat and MCF-7 cancer cell lines. BMC Complement Med Ther. 2021;21(1):22. DOI: https://doi.org/10.1186/s12906-020-03195-w
Bobo G, Davidov G, Arroqui C, Vírseda P, Marín M, Navarro M. Intra-laboratory validation of microplate methods for total phenolic content and antioxidant activity on polyphenolic extracts, and comparison with conventional spectrophotometric methods. J Sci Food Agric. 2015;95(1):204-9. DOI: https://doi.org/10.1002/jsfa.6706
Coronado J, Carrasco R, Reategui O, Toscano E, Valdez E, Zimic M, et al. Inhibitory activity against α-amylase and α-glucosidase by phenolic compounds of quinoa (Chenopodium quinoa Willd.) and cañihua (Chenopodium pallidicaule Aellen) from the Andean region of Peru. Pharmacogn J. 2021;13(4):896-901. DOI: https://doi.org/10.5530/pj.2021.13.115.
Huamanteca Manrique, Rodríguez Rodríguez. Efecto antiinflamatorio del gel a base del extracto hidroalcohólico de las hojas de Ambrosia arborescens Mill (Marco) en ratas albinas. [Trabajo de grado]. [Lima, Perú]: Universidad Interamericana para el Desarrollo. 2020. 81 p.
Rodríguez C, Gabriela J. Actividad antioxidante y antimicrobiana de los extractos preparados con diferentes solventes de las hojas de Ambrosia arborescens (Marco). [Trabajo de grado]. [Quito, Ecuador]: Universidad Central del Ecuador. 2020. 65p.
Tufinio Miranda K, Ames Canchaya H, Vergara Sotomayor A, Fukusaki Yoshizawa A, Paucar Cuba K, Tufinio Miranda K, et al. Determinación de la actividad antioxidante de extractos de hojas de Buddleja incana, Oreocallis grandiflora y Chuquiraga spinosa. Rev Soc Quím Perú. 2021;87(2):107-19. DOI: https://doi.org/10.37761/rsqp.v87i2.343.
Bhat RA, Hakeem KR, Dervash MA. Phytomedicine: A Treasure of Pharmacologically Active Products from Plants. 1st ed. Amsterdam: Elsevier; 2021. 776p.
Karabegović IT, Stojičević SS, Veličković DT, Todorović ZB, Nikolić NČ, Lazić ML. The effect of different extraction techniques on the composition and antioxidant activity of cherry laurel (Prunus laurocerasus) leaf and fruit extracts. Ind Crops Prod. 2014;54:142-8. DOI: https://doi.org/10.1016/j.indcrop.2013.12.047
Oreopoulou A, Tsimogiannis D, Oreopoulou V. Extraction of polyphenols from aromatic and medicinal plants: An overview of the methods and the effect of extraction parameters. In: Watson RR, editor. Polyphenols in plants. 2nd ed. London: Academic Press; 2019. p. 243-59. DOI: https://doi.org/10.1016/B978-0-12-813768-0.00025-6
Dirar AI, Alsaadi DHM, Wada M, Mohamed MA, Watanabe T, Devkota HP. Effects of extraction solvents on total phenolic and flavonoid contents and biological activities of extracts from Sudanese medicinal plants. S Afr J Bot. 2019;120:261-7. DOI: https://doi.org/10.1016/j.sajb.2018.07.003
Plaskova A, Mlcek J. New insights into the application of water or ethanol-water plant extracts rich in active compounds in food. Front Nutr. 2023;10:1118761. DOI: https://doi.org/10.3389/fnut.2023.1118761
Martino VS, Sülsen VP. Lactonas sesquiterpénicas: Promisorio grupo de compuestos naturales bioactivos. Academia Nacional de Farmacia y Bioquímica. 2019. Available from: https://ri.conicet.gov.ar/bitstream/handle/11336/120348/CONICET_Digital_Nro.8907dbe1-fdce-40d9-b45b-b1c67d12cdf0_A.pdf?sequence=2&isAllowed=y.
Orlando J, Molina R, Maricela A, Castellano T, Ramiro E, Carvajal C, et al. Extracción hidroalcohólica de polifenoles a partir de las hojas de cedrón (Aloysia citrodora Paláu) como ingrediente alimentario natural. Rev Recur Nat Prod Sust. 2022;1(2):56-69. Available from: http://investigacion.utc.edu.ec/index.php/RENPYS/article/view/449/613
Llanga Guamán BG. Determinación de la actividad antioxidante de los extractos de quishuar (Buddleja incana), aliso (Alnus acuminata) y romerillo (Hypericum laricifolium) localizados en tres zonas geográficas diferentes. [Trabajo de grado]. [Riobamba, Ecuador]: Escuela Superior Politécnica de Chimborazo; 2014. 106 p.
Kazan A, Koyu H, Turu IC, Yesil-Celiktas O. Supercritical fluid extraction of Prunus persica leaves and utilization possibilities as a source of phenolic compounds. J Supercrit Fluids. 2014;92:55-9. DOI: https://doi.org/10.1016/j.supflu.2014.05.006.
30. Kazan A, Koyu H, Turu IC, Yesil-Celiktas O. Supercritical fluid extraction of Prunus persica leaves and utilization possibilities as a source of phenolic compounds. J Supercrit Fluids. 2014;92:55-9. DOI: https://doi.org/10.1016/j.supflu.2014.05.006
Turvey T, Lall N. Anti-proliferative properties of various South African Buddleja species. In: Máthé Á, editor. Medicinal plants for cosmetics, health and diseases. Boca Raton: CRC Press; 2022. p. 175-98. DOI: https://doi.org/10.1201/9781003108375-10
Silva-Correa CR, Villarreal-La Torre VE, González-Siccha AD, Cruzado-Razco JL, González-Blas MV, Sagástegui-Guarniz WA, et al. Acute toxicity of aqueous extract of Ambrosia arborescens Mill. on biochemical and histopathological parameters in rats. Toxicol Res. 2022;38(2):225-33. DOI: https://doi.org/10.1007/s43188-021-00106-0
Tammar S, Salem N, Aidi Wannes W, Limam H, Bourgou S, Fares N, et al. Chemometric profiling and bioactivity of verbena (Aloysia citrodora) methanolic extract from four localities in Tunisia. Foods. 2021;10(12):2912. DOI: https://doi.org/10.3390/foods10122912
Caturano A, D’Angelo M, Mormone A, Russo V, Mollica MP, Salvatore T, et al. Oxidative stress in type 2 diabetes: Impacts from pathogenesis to lifestyle modifications. Curr Issues Mol Biol. 2023;45(8):6651. DOI: https://doi.org/10.3390/cimb45080420.
Chen X, Xie N, Feng L, Huang Y, Wu Y, Zhu H, et al. Oxidative stress in diabetes mellitus and its complications: From pathophysiology to therapeutic strategies. Chin Med J (Engl). 2024. DOI: 10.1097/CM9.0000000000003230.
Ávila-Reyes JA, Almaraz-Abarca N, Alvarado EAD, Torres-Ricario R, Naranjo-Jiménez N, Gutierrez-Velazquez MV, et al. α-Glucosidase and α-amylase inhibition potentials of ten wild Mexican species of Verbenaceae. Trop J Pharm Res. 2019;18(1):31-6. DOI: https://doi.org/10.4314/tjpr.v18i1.5
Bhosale HJ, Mamdapure SV, Panchal RB, Dhuldhaj UP. α-Amylase, α-glucosidase and aldose reductase inhibitory and molecular docking studies on Tinospora cordifolia (Guduchi) leaf extract. Future J Pharm Sci. 2024;10(1):1-13. DOI: https://doi.org/10.1186/s43094-024-00671-9
Elhady SS, Youssef FS, Alahdal AM, Almasri DM, Ashour ML. Anti-hyperglycaemic evaluation of Buddleia indica leaves using in vitro, in vivo and in silico studies and its correlation with the major phytoconstituents. Plants. 2021;10(11):2351. DOI: https://doi.org/10.3390/plants10112351
Kulkarni AA, Kamble RP. α-Amylase inhibitory secondary metabolites from Artemisia pallens Wall. ex DC: Biochemical and docking studies. Biol Life Sci Forum. 2022;11(1):73. DOI: https://doi.org/10.3390/IECPS2021-11978.
Kidane Y, Bokrezion T, Mebrahtu J, Mehari M, Gebreab YB, Fessehaye N, et al. In vitro inhibition of α-amylase and α-glucosidase by extracts from Psiadia punctulata and Meriandra bengalensis. Evid Based Complement Alternat Med. 2018;2018:1-8. DOI: 10.1155/2018/2164345.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Irvin Tubon, Erick Cunalata , Goering Octavio Zambrano-Cárdenas , Jessica Paola Arcos-Logroño, Gabriela Liseth Vaca Altamirano

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Copyright Notice and Open Access Statement
The Journal Vitae works under the Open Access license, and the published manuscripts remain available for the public, both on the Journal's website and in databases, under the Creative Commons license, "Noncommercial Attribution" and "Share alike" systems, adopted in Colombia. Hence, when the authors agree to publish in the Journal Vitae, they will not have the right to economic retributions on publications and reproductions through different diffusion media. The documents are freely available to the internet public, permitting users to read, download, copy, distribute, print, search, or link to the full texts and pass them as data to software. The only constraint on reproduction and distribution, should be to give authors control over the integrity of their work and the right to be appropriately acknowledged and cited.
Authors declare that:
-
They are the intellectual property owners and are responsible for all the information stated in the article.
-
This manuscript has not been submitted or published in other printed or digital media. They accept the responsibility for the judgments, opinions, and points of view expressed in the published article and, therefore, they exonerate Universidad de Antioquia and Journal Vitae from any process.
-
They exempt Universidad de Antioquia and Journal Vitae from settling conflicts or disputes related to the authorship of the referred article.
-
They accept the revision of the original manuscript by suitable personnel, and they bind themselves to perform the corrections appointed or suggested by the assessors.
-
Therefore, they know the editorial process and will not bind the Editorial Board of the Journal to assume any obligations regarding the volume and issue in which the article is published.
-
They transfer the rights of publication, reprinting, and distribution of the article from the moment of its approval, in print and digital format, without the right to economic rewards, and under the licensing conditions considered relevant by Journal Vitae.
-
They fully authorize Universidad de Antioquia and Journal Vitae to submit the published material to the diverse databases and indexing systems where the Journal can be found to comply with the requirements of the regulatory authorities to maintain the national classification of journals.
-
They will assume the article publication costs established for the current issue, and they will make the payment as soon as they are informed about the volume and the issue in which the final version of the article is published.
-
After the article is published, you can share digital or printed copies in a noncommercial manner. You will be able to use the paper in your institution or company for educational or research purposes, including the use in course programs.
Conflict of interest: Authors are responsible for recognizing and disclosing any financial or other benefits that could be perceived to bias their work, acknowledging all financial support and any personal connections with potential sponsors. Examples of such conflicts include receiving research funds or honoraria, serving on advisory boards, stock ownership, or employment and consulting arrangements. Authors without such connections should clearly state that they have no financial support or personal relationships that could be perceived to bias their work. All conflicts of interest should be disclosed on the author's identification page of the manuscript.









