In-vitro effect of the methanolic extract of Morinda citrifolia against the life cycle of Dermatobia hominis
DOI:
https://doi.org/10.17533/udea.vitae.v31n3a353998Keywords:
cattle, myiasis, parasite control, phytotherapyAbstract
Background: bovine cutaneous dermatobiosis or furuncular myiasis caused by Dermatobia hominis is a parasitosis that mainly affects bovines in the tropics and represents a particular interest in public health as zoonosis. Its control is based on ivermectins, which have long withdrawal times, affecting the productive dynamics within dairy cattle herds.
Objective: to assess the in-vitro effect of the methanolic extract of the M. citrifolia ripe fruit against the life cycle of D. hominis.
Methods: D. hominis larvae were taken directly from naturally parasitized bovine skins. These larvae were exposed by immersion to different concentrations of the methanolic extract of M. citrifolia (10, 50, 100, 200, 300, 400, 460 mg/mL) diluted in distilled water. Ivermectin 1% was used as a positive control, and distilled water as a negative control. Subsequently, the larvicidal activity was evaluated in the first 48 hours post-immersion (PI), the pupicidal activity within 10 to 23 days PI, and the inhibition of the imagos emergence as well as their anatomical alterations, were evaluated within 24 to 35 days PI; recreating the pupal development and their hatching in the soil under controlled laboratory conditions. CL50 and CL90 for the larvae phase were estimated through Probit regression analysis.
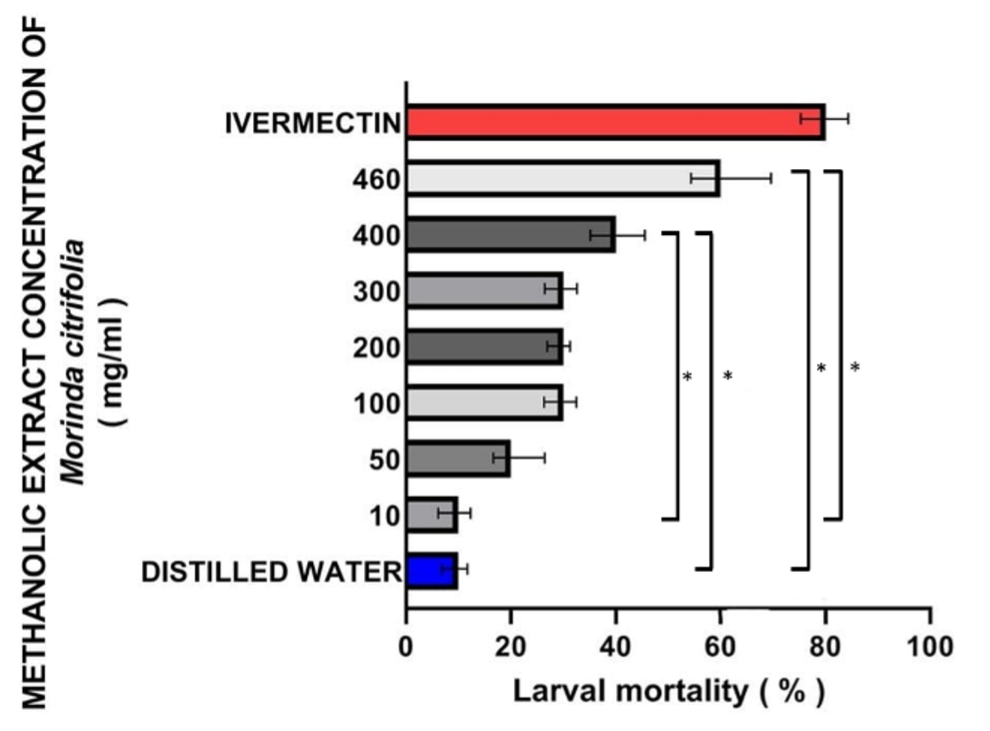
Results: M. citrofolia concentrations of 400 and 460 mg/mL had a significant (p<0.05) larvicidal effect of 40% (95% CI 34.7 - 43.9) and 60% (95% CI 56.8 - 67.3), respectively. The pupicidal effect on the surviving larvae was significant (p<0.05) at 300, 400, and 460 mg/mL: 40% (95% CI 37.9 - 42.3), 60% (95% CI 55.7 - 65.9) and 70% (CI 95% 67.1 – 76.7), respectively. The inhibition of the emergence of imagos was significant (p<0.05) 50% (95% CI 42.3 - 57.8) in all concentrations equal to or greater than 200 mg/mL. Finally, 20% (95% CI 12.6 - 29.3) of the emerging imagos at 460 mg/mL presented morphoananomic alterations (p<0.05). The LC50 and LC90 estimated (larval phase) were 22.36 mg/mL (95%CI 15.06-33.19) and 245.08 mg/mL (95%CI 165.10-363.82), respectively.
Conclusions: The methanolic extract of M. citrifolia was effective as larvicide, altering the pupation and the emergence of imagos of D. hominis. In addition, it modified the imagos morphoanatomy; interesting results to promote in-situ and other bioguided fractionation studies of this extract in different D. hominis stages; being M. citrifolia a plant species widely adapted to the conditions of the Meta department, Colombia.
Downloads
References
Mateus JG. Dermatobia hominis (L. Jr. 1781): un problema del ganado bovino en Centro y Sur América. Agrosavia [Internet]. 1983 [cited 2024 dic 2]; 9: 2821-32. Avalilable from: https://repository.agrosavia.co/bitstream/handle/20.500.12324/29669/27128_15144.pdf?sequence=1&isAllowed=y
Grisi L, Cerqueira R, de Souza J, Medeiros A, Andreotti R, Duarte P, et al. Reassessment of the potential economic impact of cattle parasites in Brazil. Braz. J. Vet. Parasitol. 2014;23(2):150-156. DOI: http://dx.doi.org/10.1590/S1984-29612014042
Cañueto J, Roncero M, de Unamuno P. Miasis foruncular: una dermatosis emergente en países desarrollados. Piel. 2010;25:146-151. DOI: http://dx.doi.org/10.1016/j.piel.2009.11.005.
Bernhardt V, Finkelmeier F, Verhoff M, Amendt J. Myiasis in humans - a global case report evaluation and literature analysis. Parasitol Res. 2019;118:389-397. DOI: https://doi.org/10.10 07/s0 0436- 018- 6145- 7
Gomes P, Koller W, Gomes A, Carvalho J, Zorzatto J. Dípteros fanídeos vetores de ovos de Dermatobia hominis em Campo Grande, Mato Grosso do Sul. Pesquisa Veterinária Brasileira. 2002;22(3):114-118. DOI: https://doi.org/10.1590/S0100-736X2002000300005
Castaño F, Blanco R, Gómez V, Cardona J, Montes J. Frequência da dermatobiose cutânea bovina em vacas da raça Holandesa de uma granja leiteira de Viçosa (MG, Brasil). Ces. Med. Vet. Zootec [Internet]. 2014 [cited 2024 dic 2]; 8(1):82-94. Available from: https://revistas.ces.edu.co/index.php/mvz/article/view/2835
Barbosa C, Sanavria A, Passos M, Barbosa RC. Fase parasitária e alterações clínicas em bovinos infestados experimentalmente com larvas de Dermatobia hominis (Diptera: Cuterebridae). Parasitol Latinoam [Internet]. 2002 [cited 2024 dic 2]; 57: 15-20. Available from: https://www.scielo.cl/pdf/parasitol/v57n1-2/art05.pdf
Sanavria A, Barbosa C, Bezerra E, Morais M, Giupponi P. Distribuição e frequência de larvas de Dermatobia hominis (Linnaeus Jr., 1781) (Diptera: Cuterebridae) em peles de bovinos. Parasitol Latinoam. 2002;57(1-2):21-4. DOI: http://dx.doi.org/10.4067/S071777122002000100006
Ministerio de Agricultura y desarrollo Rural, Resolución número 00072 de 2007. https://www.minagricultura.gov.co/Normatividad/Resoluciones/Resoluci%C3%B3n%20No%20000072%20de%202007.pdf . 2007 (accessed 30 may 2021).
Failoc V, Molina C, Salazar J, Samamé A, Silva H. Case report: Myiasis due to Cochliomyia hominivorax and Dermatobia hominis: clinical and pathological differences between two species in Northern Peru. Am. J. Trop. Med. Hyg. 2018;98(1):150–153. DOI: https://doi.org/10.4269/ajtmh.16-0437
Barbosa C, Sanavria A, Barbosa M. Fase parasitária e alterações clínicas em bovinos infestados experimentalmente com larvas de Dermatobia hominis (Diptera: Cuterebridae). Parasitol Latinoam. 2002; 57(1): 15-20. DOI: http://dx.doi.org/10.4067/S0717-77122002000100005
López L. Furuncular myiasis with multiple inoculation sites from the larva of Dermatobia hominis. Actas Dermo-Sifiliográficas 2004;95(10):633-634. DOI: https://doi.org/10.1016/S0001-7310(04)76901-X
Moltó J, Molina M, Mengual E, Hueso J. External ophthalmomyiasis due to Dermatobia hominis. A case report. Archivos de la Sociedad Española de Oftalmología 2018;93(8):402-405. DOI: https://doi.org/10.1016/j.oftale.2018.05.009
Vikram A, Gjerde H, Mailman T, Archibald C. Ocular myiasis secondary to Dermatobia hominis. Canadian Journal of Ophthalmology. 2020;55(4):e139- e140. DOI: https://doi.org/10.1016/j.jcjo.2020.01.004
Nieto L, Rodriguez E, Martin P, Pulido A. First report of Autochthonous Furuncular Myiasis caused by Dermatobia Hominis in Europe. Journal of Infection. 2021;83(1):119-145. DOI: https://doi.org/10.1016/j.jinf.2021.02.005
Neves J, Carvalho N, Amarante A. Dermatobia hominis: Potencial risk of resistance to macrocyclic lactones. Veterinary Parasitology. 2015;212:483-486. DOI: https://doi.org/10.1016/j.vetpar.2015.06.029
Conde H, Gonçalves D, Green de Freitas M, Cabrera da Silva M, Almeida Fernando. First report of Dermatobia hominis resistant to doramectin in cattle. Veterinary Parasitology. 2021;(289)109335. DOI: https://doi.org/10.1016/j.vetpar.2020.109335
Lumaret J, Martínez I. El impacto de productos veterinarios sobre insectos coprófagos: consecuencias sobre la degradación del estiércol en pastizales. Acta Zool. Mex [Internet]. 2005 [cited 2023 may 21];21(3):137-148. Available from: http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S0065-17372005000300007&lng=es
Cova A, Azevedo A, Gheyi H , Ribas R, de Oliveira L, Menezes R. Gas exchange, Chlorophyll Fluorescence and Pigments of Noni (Morinda citrifolia L.) under Salt Stress. Journal of Agricultural Science. 2018;10(2):1916. DOI: https://doi.org/10.5539/jas.v10n2p318.
Legal L, Chappe B, Jallon J. Molecular basis of Morinda citrifolia (L.): Toxicity on Drosophila. J Chem Ecol. 1994;20(8):1931-43. DOI: http://dx.doi.org/10.1007/BF02066234
Nayak S, Mengi S. Actividad inmunoestimulante de los extractos y bioactivos de los frutos deMorinda citrifolia. Biología Farmacéutica. 2009; 473, 248-254. DOI: http://dx.doi.org/10.1080/13880200802435697
Trieu L, Minh T, Pham N, Thi K, Thi P, Xu K et al. Phytochemical Analysis and Wound-Healing Activity of Noni (Morinda citrifolia) Leaf Extract Journal of Herbs. 2020; 26(4):379-393, DOI: http://dx.doi.org/10.1080/10496475.2020.1748159
Sanabria GA. Análisis fitoquímico preliminar: Metodología y su aplicación en la evaluación de 40 plantas de la familia Compositae. [Grade Work]. [Bogotá, Colombia]: Universidad Nacional de Colombia: 1983. 77p
Pires F, Moya Borja G, Barreira J, Pinho R, Alves C. The main proteinases in Dermatobia hominis second and third instars larvae are serine-proteinases. Vet Parasitol. 2007;30 (145):326-31. DOI: https://doi.org/10.1016/j.vetpar.2007.01.001
Paéz R, Villa L. Identificación de larvas productoras de miasis obtenidas del cepario de la Universidad Colegio Mayor de Cundinamarca con importancia en salud pública. Nova. 2017; 15(28), 79-91. DOI: https://doi.org/10.22490/24629448.2082
Francesconi F, Lupi O. Myiasis. Clin Microbiol Rev. 2012;25:79. DOI: https://doi.org/10.1128/CMR.00010-11
Gomes A. Efectividad de cialotrina dipermetrin, cipotrin, decametrina y flumetrina en larvas de Dermatobia hominis. (L.Jr., 1781). [Grade Work]. [Porto Alegre, Brasil]: UFRGS: 1984. 40 p.
Castro W, de Souza Martins J, Menezes de Souza H, Heinzen H, Cesio M, Mato M et al. Toxicity of Piper aduncum L. (Piperales: Piperaceae) from the Amazon forest for the cattle tick Rhipicephalus (Boophilus) microplus (Acari: Ixodidae). Veterinary Parasitology. 2009;64(2-4):267-274. DOI: https://doi.org/10.1016/j.vetpar.2009.06.006
Zeledón R. Algunas observaciones sobre la biología de la Dermatobia hominis (L. Jr.) y el problema del tórsalo en Costa Rica. Revista de Biología Tropical [Internet]. 2009 [cited 2024 dic 2];5(1):63-75. Available from: https://tropicalstudies.org/rbt/attachments/volumes/vol5-1/05-Zeledon-Dermatobia.pdf
Cadena J, Valles J. El nuche: vida y control. Revista ICA Informa. [Internet] 1973 [cited 2024 dic 2];8(5):5-6. Available from: https://repository.agrosavia.co/handle/20.500.12324/14198
Moya G. Analítico: Estudios sobre la biología, morfología y esterilización del tórsalo, Dermatobia hominis (L., Jr.). [Grade Work]. [Turrialba, Costa Rica]: Instituto Interamericano de Ciencias agrícola de la OEA: 1966. 55 p.
Rezaeilaal A, Nasoori H, Sadat H, Samanian A, Qavami N, Momtaz S, et al. Traditional medicine and natural products as antiparasitic agents. In: Gupta N, Kesharwani P, editors. Advances in antiparasitic therapies and drug delivery. Cambridge: Academic Press.; 2024. Chapter 12. pp. 33–90.
Sadino A, Levita J, Saptarini N, Fristiohady A. An evidence-based review of Morinda citrifolia L. (Rubiaceae) fruits on animal models, human studies, and case reports. Journal of Pharmacy & Pharmacognosy Research. 2024;12(3). DOI: https://doi.org/10.56499/jppres23.1832_12.3.391
Arunachalam V. Morinda citrifolia L. (Rubiaceae): a multi-purpose tree for coastal ecosystems and its variability in Konkan region of India. Genetic Resources and Crop Evolution. 2018;65(6):1751-1765. DOI: https://doi.org/10.1007/s10722-018-0642-5
Algenstaedt P, Stumpenhagen A, Westendorf J. The Effect of Morinda citrifolia L. Fruit Juice on the Blood Sugar Level and Other Serum Parameters in Patients with Diabetes Type 2. Evid Based Complement Alternat Med. 2018;3565427. DOI: https://doi.org/10.1155/2018/3565427
Yancey J, Apple J, Kegley E, Godbee R. Effects of Morinda citrifolia (Noni) pulp on growth performance and stress responses of growing cattle. Prof. Anim. Sci. 2013;29:420–425. DOI: https://doi.org/10.15232/S1080-7446(15)30255-2
Sunder J, Jeyakumar S, Sujatha T, Kundu A. Grommune: Morinda citrifolia- based herbal tonic for growth and immunity for commercial broilers. J. Appl. Anim. Res. 2015;43(2):137–140. DOI: https://doi.org/10.1080/09712119.2014.928628
Singh D. Morinda citrifolia L. (Noni): a review of the scientific validation for its nutritional and therapeutic properties. J. Diabet. Endocrinol. 2012;3(6):77–91. DOI: https://doi.org/10.5897/JDE10.006
Nitteranon V, Zhang G, Darien B, Parkin K. Isolation and synergism of in vitro anti-inflammatory and quinone reductase (QR) inducing agents from the fruits of Morinda citrifolia (noni). Food Res Int. 2011;44:2271-2277. DOI: http://doi.org/10.1016/j.foodres.2010.11.009.
Kusirisin W, Srichairatanakool S, Lerttrakarnnon P, Lailerd N, Suttajit M, Jaikang C, et al. Antioxidative activity, polyphenolic content and anti-glycation effect of some Thai medicinal plants traditionally used in diabetic patients. Med Chem. 2009;5(2):139-47. DOI: http://doi.org/10.2174/157340609787582918.
Dalcin M, Oliveira E, Santos G. Morinda citrifolia Essential Oil: A Plant Resistance Biostimulant and a Sustainable Alternative for Controlling Phytopathogens and Insect Pests. Biology (Basel). 2024;13(7):479. DOI: https://www.mdpi.com/2079-7737/13/7/479
Almeida F, de Oliveira A, Abreu L, da Silva K. In vitro activity of Morinda citrifolia Linn. fruit juice against the axenic amastigote form of Leishmania amazonensis and its hydrogen peroxide induction capacity in BALB/c peritoneal macrophages. BMC Res Notes. 2018;11(1):492. DOI: http://doi.org/10.1186/s13104-018-3555-7
Hwei J, Waiho K, Fazhan H, Shaibani N, Manan H, Yik-Sung Y et al. Effect of Noni, Morinda citrifolia fruit extract supplementation on the growth performances and physiological responses of the hepatopancreas of Whiteleg shrimp, Penaeus vannamei Post Larvae. Aquaculture Reports. 2021;21. DOI: https://doi.org/10.1016/j.aqrep.2021.100798
Arteaga M, Pérez J, González C, Barro A, González B. Clasificación toxicológica aguda del fruto seco pulverizado de Morinda citrifolia L. (NONI-C)® en ratas Cenp: SPRD. Rev Cubana Plant Med [Internet] 2009 [cited 2024 dic 2];14(4). Available from: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S1028-47962009000400006&lng
Jayakumar S, Arumugam R, Sathiskumar S, Pugazhenthi M. Herbal medicine as a live practice for treating livestock ailments by indigenous people: A case study from the Konar community of Tamil Nadu. South African Journal of Botany. 2018;118:23-32. DOI: https://doi.org/10.1016/j.sajb.2018.06.002
Iqbal Z, Jabbar A, Akhtar M, Ghulam M, Lateef M. Review Possible Role of Ethnoveterinary Medicine in Poverty Reduction in Pakistan: Use of Botanical Anthelmintics as an Example. Journal Of Agriculture & Social Sciences [Internet] 2005 [cited 2024 dic 2];1(2):187–195. Available from: https://www.researchgate.net/publication/228371961_Possible_role_of_Ethnoveterinary_medicine_in_poverty_reduction_in_Pakistan_Use_of_botanical_Anthelmintics_as_an_example
Nápoles V, Sebasco R, Colas M, López S, Meireles R. Eficacia in vitro de Morinda citrifolia L para el control de Rhipicephalus (Boophilus) microplus (Acari: Ixodidae). Rev. investig. vet. Perú. 2016;27(4):833-839. DOI: http://dx.doi.org/10.15381/rivep.v27i4.12562.
Botello R, Botello L, Borroto N, Suárez M, Pérez A, Rodríguez Y, et al. Control de garrapatas Riphicephalus (Boophilus) microplus en bovinos con el inmunógeno Herber biogar. Redvet [Internet] 2011 [cited 2024 dic 2];12(5):1-10. Available from: https://www.redalyc.org/pdf/636/63622168003.pdf
Valencia G, Ruíz J, Avendaño A, Ramírez J. Evaluation of a product to base of cipermetrina clorpirifos on the larvas of Dermatobia hominis in bovine, in the municipality of Titiribí, Antioquia. CES Medicina Veterinaria y Zootecnia [Internet] 2007 [cited 2023 Jun 2];2(1):21-27. Available from: https://www.redalyc.org/pdf/3214/321428097002.pdf
Florez A, Martinez J, Solano J, Pinilla C. First report of furuncular myiasis in a domestic dog caused by Dermatobia hominis (Linnaeus, 1781) in Colombia. Veterinary Parasitology: Regional Studies and Reports. 2020;20:100402. DOI: https://doi.org/10.1016/j.vprsr.2020.100402.
Leite C, Umehara O, Oliveira P, Facury E, Okano H. Efficacy and persistence of doramectin and ivermectin against natural infestations of Dermatobia hominis larvae in cattle. Rev. Bras. Parasitol. Vet [Internet]. 1999 [cited 2024 dic 2]; 8(2):161-166. Available from: http://www.ufrrj.br/rbpv/821999/c82161_166.pdf
Muñoz-Rivas G. Notas sobre Dermatobia hominis. Revista de la Facultad de Medicina Veterinaria y de Zootecnia [Internet]. 1956 [cited 2024 dic 2]. Available from: https://revistas.unal.edu.co/index.php/remevez/article/view/66935
Sánchez-Varela A, Rodríguez-Luna I. Mortalidad de larvas de Spodoptera frugiperda por efecto de extractos de fruto de Morinda citrifolia l. (noni). Rev Bol Quím [Internet]. 2017 [cited 2024 dic 2]; 34(5):138-141. Available from: http://www.scielo.org.bo/scielo.php?script=sci_arttext&pid=S0250-54602017000500002&lng=es
Kovendan K, Murugan K, Shanthakumar S, Vincent S. Evaluation of larvicidal and pupicidal activity of Morinda citrifolia L. (Noni) (Family: Rubiaceae) against three mosquito vectors. Asian Pac. J. Trop. Dis. 2012;2(1):S362–S369. DOI: https://doi.org/10.1016/S2222-1808(12)60182-0.
Legal L, David J, Jallon J. Toxicity and attraction effects produced by Morinda citrifolia fruits on the Drosophila melanogaster complex of species. Chemoecology 1992;3:125–129. DOI: https://doi.org/10.1007/BF01370140
Esparza-Díaz G, López-Collado J, Villanueva-Jiménez J, Osorio-Acosta F, Otero-Colina G, Camacho-Díaz E. Concentración de azadiractina, efectividad insecticida y fitotoxicidad de cuatro extractos de Azadirachta indica A. Juss. Agrociencia [Internet]. 2010 [cited 2024 dic 2]; 44(7):821-833. Available from: http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S1405-31952010000700008&lng=es&tlng=es
Kovendan K, Shanthakumarb S, Praseejac C, Kumara P, Murugana K, Vincentd S. Mosquitocidal properties of Morinda citrifolia l. (Noni) (family: Rubiaceae) leaf extract and Metarhizium anisopliae against malaria vector, Anopheles stephensi Liston. (Diptera: Culicidae). Asian Pac. J. Trop. Dis. 2014;4(1):S173–S180. DOI http://dx.doi.org/10.1016/S22221808(14)60435-7
Opoku-Bamfoh O., Kwarteng S., Owusu F., Akpanya R., Mensah K., Badu M. Repellent and larvicidal properties of selected indigenous plants in the control of Anopheles mosquitoes. Journal of Vector Borne Diseases. 61(1):90-100. DOI: https://journals.lww.com/jvbd/fulltext/2024/61010/repellent_and_larvicidal_properties_of_selected.10.aspx
Rahayu R, Ayunda P, Henny H, Jannatan R. Efficacy of Noni (Morinda citrifolia L.) Ethanolic Leaf Extract Against German Cockroach (Blattella germanica L.). Pak J Biol Sci. 2021;24(5):629- 63. DOI: http://doi.org/10.3923/pjbs.2021.629.635.
Morales J, Castillo J, Luna I. Aceite esencial del fruto del noni (Morinda citrifolia: rubiaceae) como larvicida del mosquito Aedes aegypti (díptera: Culicidae). Tecnociencia [Internet]. 2010 [cited 2024 dic 2]; 12(1):53-64. Available from: https://core.ac.uk/download/pdf/354264478.pdf
Instituto Colombiano Agropecuario (ICA). Censo Pecuario Nacional. https://www.ica.gov.co/areas/pecuaria/servicios/epidemiologia-veterinaria/censos-2016/censo-2018 2021 (Accessed 2 dic 2024).
Sheikh M, Ahamad H, Haq E, Ahamad B, Khan Z, Rizi, R. Survey ofethno-veterinary medicine amongst ethno-practitioners of western Uttar-Pradesh province of India. Journal of Medicinal Plant Research. 2013;7(9):509–516. DOI: https://doi.org/10.5897/JMPR012.1121
Wall R, Shearer D. Veterinary ectoparasites: biology, pathology and control, second edition, Blackwell Science, Osney; 2001. 271 p.
Pérez de León A, Mitchell R, Watson D. Ectoparasites of Cattle. Veterinary Clinics of North America: Food Animal Practice. 2020;36(1):173-185. DOI: https://doi.org/10.1016/j.cvfa.2019.12.004
Daulai M., Wijayanti I., Retnani Y. Nano emulsion Formulation of Noni Leaf Extract and Maggot Oil (Hermetia illucens) as an Alternative to Antibiotic Growth Promoters. Archives of Razi Institute Journal. 2024;79(4):741-748. HTTP: https://archrazi.areeo.ac.ir/article_130668.html.
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Dumar Alexander Jaramillo Hernández, Rafael Felipe Quevedo Carrillo, Diego Arnaldo Cadena Franco, Angélica Elizabeth Gonzalez Reina, Lida Carolina Lesmes-Rodríguez, Luz Natalia Pedraza-Castillo

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Copyright Notice and Open Access Statement
The Journal Vitae works under the Open Access license, and the published manuscripts remain available for the public, both on the Journal's website and in databases, under the Creative Commons license, "Noncommercial Attribution" and "Share alike" systems, adopted in Colombia. Hence, when the authors agree to publish in the Journal Vitae, they will not have the right to economic retributions on publications and reproductions through different diffusion media. The documents are freely available to the internet public, permitting users to read, download, copy, distribute, print, search, or link to the full texts and pass them as data to software. The only constraint on reproduction and distribution, should be to give authors control over the integrity of their work and the right to be appropriately acknowledged and cited.
Authors declare that:
-
They are the intellectual property owners and are responsible for all the information stated in the article.
-
This manuscript has not been submitted or published in other printed or digital media. They accept the responsibility for the judgments, opinions, and points of view expressed in the published article and, therefore, they exonerate Universidad de Antioquia and Journal Vitae from any process.
-
They exempt Universidad de Antioquia and Journal Vitae from settling conflicts or disputes related to the authorship of the referred article.
-
They accept the revision of the original manuscript by suitable personnel, and they bind themselves to perform the corrections appointed or suggested by the assessors.
-
Therefore, they know the editorial process and will not bind the Editorial Board of the Journal to assume any obligations regarding the volume and issue in which the article is published.
-
They transfer the rights of publication, reprinting, and distribution of the article from the moment of its approval, in print and digital format, without the right to economic rewards, and under the licensing conditions considered relevant by Journal Vitae.
-
They fully authorize Universidad de Antioquia and Journal Vitae to submit the published material to the diverse databases and indexing systems where the Journal can be found to comply with the requirements of the regulatory authorities to maintain the national classification of journals.
-
They will assume the article publication costs established for the current issue, and they will make the payment as soon as they are informed about the volume and the issue in which the final version of the article is published.
-
After the article is published, you can share digital or printed copies in a noncommercial manner. You will be able to use the paper in your institution or company for educational or research purposes, including the use in course programs.
Conflict of interest: Authors are responsible for recognizing and disclosing any financial or other benefits that could be perceived to bias their work, acknowledging all financial support and any personal connections with potential sponsors. Examples of such conflicts include receiving research funds or honoraria, serving on advisory boards, stock ownership, or employment and consulting arrangements. Authors without such connections should clearly state that they have no financial support or personal relationships that could be perceived to bias their work. All conflicts of interest should be disclosed on the author's identification page of the manuscript.









