Comparative in vitro evaluation of a biosimilar enoxaparin candidate and its reference product
DOI:
https://doi.org/10.17533/udea.vitae.v32n3a361315Keywords:
Biosimilar, Enoxaparin, Anticoagulation, Low molecular weight heparin, ThromboelastometryAbstract
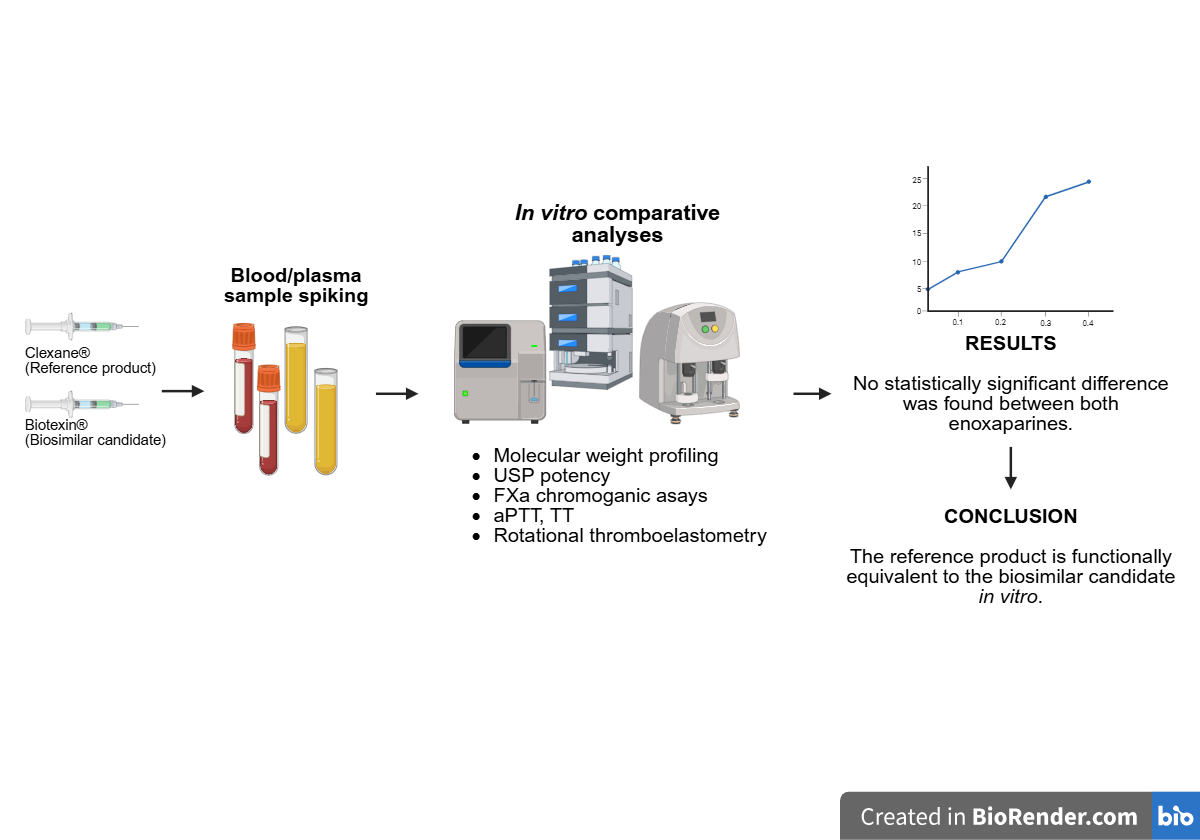
BACKGROUND: Enoxaparin, a low molecular weight heparin, has been widely used to prevent and treat thromboembolic disorders. Following the expiration of the patent of the reference product, biosimilar alternatives were developed, requiring thorough comparability assessments to ensure efficacy and safety. OBJECTIVE: To compare the in vitro pharmacodynamic and structural properties of Biotexin® and the reference product Clexane®. METHODS: The products were evaluated using anti-FXa chromogenic assays, clot-based tests (aPTT, TT), and whole-blood thromboelastometry (ROTEM). Structural analyses included molecular weight profiling and USP potency determination. RESULTS: Biotexin® showed anti-FXa activity comparable to that of Clexane®, with overlapping EC₅₀ confidence intervals. No statistically significant differences were observed in aPTT or TT assays (P = 0.499 and P = 0.538, respectively). ROTEM analysis confirmed a significant prolongation of clotting time (CT) and clot formation time (CFT) compared to the control (P = 0.0043 and P = 0.0064, respectively), with no differences between the two products. Molecular weight distribution and USP potency were also comparable. CONCLUSION: These findings confirm the in vitro biosimilarity between Biotexin® and the reference product, supporting their functional equivalence and justifying further pharmacokinetic and clinical studies to establish therapeutic interchangeability.
Downloads
References
Ingle RG, Agarwal AS. A world of low molecular weight heparins (LMWHs) enoxaparin as a promising moiety—A review. Carbohydr Polym 2014;106:148–53. DOI: https://doi.org/10.1016/j.carbpol.2014.01.100.
Sultana R, Kamihira M. Multifaceted Heparin: Diverse Applications beyond Anticoagulant Therapy. Pharmaceuticals 2024;17:1362. DOI:https://doi.org/10.3390/ph17101362.
Saxena S, Chaudhary M, Chaudhary S, Aggarwal A. Bioequivalence of a biosimilar enoxaparin ( CLOTI‐XATM ) and its innovator (Clexane® ): a single‐dose , randomized, double‐blind , two‐period , two‐treatment , two‐sequence , crossover, balanced study in healthy human subjects. Pharmacol Res Perspect 2022;10:e00979. DOI:https://doi.org/10.1002/prp2.979.
Dziri C, Ben Hmida W, Dougaz W, Khalfallah M, Samaali I, Jerraya H, et al. Biosimilar versus branded enoxaparin to prevent postoperative venous thromboembolism after surgery for digestive tract cancer: Randomized trial. PLOS ONE 2023;18:e0293269. DOI:https://doi.org/10.1371/journal.pone.0293269.
Ramacciotti E, Ferreira U, Costa AJV, Raymundo SRO, Correa JA, Neto SG, et al. Efficacy and Safety of a Biosimilar Versus Branded Enoxaparin in the Prevention of Venous Thromboembolism Following Major Abdominal Surgery: A Randomized, Prospective, Single-Blinded, Multicenter Clinical Trial. Clin Appl Thromb 2018;24:1208–15. DOI:https://doi.org/10.1177/1076029618786583.
Fantoni C, Bertù L, Faioni EM, Froiio C, Mariani N, Ageno W. Safety and effectiveness of biosimilar enoxaparin (Inhixa) for the prevention of thromboembolism in medical and surgical inpatients. Intern Emerg Med 2021;16:933–9. DOI:https://doi.org/10.1007/s11739-020-02536-4.
Gherghescu I, Delgado-Charro MB. The Biosimilar Landscape: An Overview of Regulatory Approvals by the EMA and FDA. Pharmaceutics 2020;13:48. DOI:https://doi.org/10.3390/pharmaceutics13010048.
Iqbal Z, Sadaf S. Commercial Low Molecular Weight Heparins — Patent Ecosystem and Technology Paradigm for Quality Characterization. J Pharm Innov 2023;18:803–35. DOI:https://doi.org/10.1007/s12247-022-09665-7.
Mielke J, Jilma B, Jones B, Koenig F. An update on the clinical evidence that supports biosimilar approvals in Europe. Br J Clin Pharmacol 2018;84:1415–31. DOI:https://doi.org/10.1111/bcp.13586.
Qneibi D, Ramacciotti E, Macedo AS, Caffaro RA, Agati LB, Siddiqui F, et al. Comparative Studies on the Anticoagulant Profile of Branded Enoxaparin and a New Biosimilar Version. Clin Appl Thromb 2020;26:1076029620960820. DOI:https://doi.org/10.1177/1076029620960820.
Berkovskii AL, Sergeeva EV, Chernobrovkin MG, Zinchenko AV. Comparative Analysis of Two Enoxaparin Sodium Drugs by Heptest. Pharm Chem J 2023;56:1407–10. DOI:https://doi.org/10.1007/s11094-023-02805-4.
Bertini S, Bisio A, Torri G, Bensi D, Terbojevich M. Molecular Weight Determination of Heparin and Dermatan Sulfate by Size Exclusion Chromatography with a Triple Detector Array. Biomacromolecules 2005;6:168–73. DOI:https://doi.org/10.1021/bm049693s.
Bisio A, Mantegazza A, Vecchietti D, Bensi D, Coppa A, Torri G, et al. Determination of the Molecular Weight of Low-Molecular-Weight Heparins by Using High-Pressure Size Exclusion Chromatography on Line with a Triple Detector Array and Conventional Methods. Molecules 2015;20:5085–98. DOI:https://doi.org/10.3390/molecules20035085.
Pehlivan M, Pehlivan M, Ceylan C, Alisik M, Yis O, Bugdayci G. Analytical Performance Evaluation of Siemens Sysmex CS-2500 and Sekisui CP-3000. Clin Lab 2022;68. DOI:https://doi.org/10.7754/Clin.Lab.2021.210606.
Yis OM, Bugdayci G, Pehlivan MB, Yildiz RN, Alisik M. Analytical performance evaluation of Sysmex CS-2500 and Stago STA Compact. Blood Coagul Fibrinolysis 2020;31:324–9. DOI:https://doi.org/10.1097/MBC.0000000000000920.
Schaden E, Schober A, Hacker S, Spiss C, Chiari A, Kozek-Langenecker S. Determination of enoxaparin with rotational thrombelastometry using the prothrombinase-induced clotting time reagent. Blood Coagul Fibrinolysis 2010;21:256–61. DOI:https://doi.org/10.1097/MBC.0b013e328337014c.
Zuluaga AF, Agudelo M, Rodriguez CA, Vesga O. Application of microbiological assay to determine pharmaceutical equivalence of generic intravenous antibiotics. BMC Clin Pharmacol 2009;9:1. DOI: https://doi.org/10.1186/1472-6904-9-1.
Sanchez‐Pena P, Hulot J, Urien S, Ankri A, Collet J, Choussat R, et al. Anti‐factor Xa kinetics after intravenous enoxaparin in patients undergoing percutaneous coronary intervention: a population model analysis. Br J Clin Pharmacol 2005;60:364–73. DOI: https://doi.org/10.1111/j.1365-2125.2005.02452.x.
Declerck P, Farouk Rezk M. The road from development to approval: evaluating the body of evidence to confirm biosimilarity. Rheumatology 2017;56:iv4–13. DOI:https://doi.org/10.1093/rheumatology/kex279.
Jeske W, McGeehan E, Iqbal O, Hoppensteadt D, Walenga JM, Fareed J. Comparison of Branded Enoxaparin (Lovenox) and a Biosimilar Version of Enoxaparin (Fibrinox). Blood 2010;116:4385–4385. DOI:https://doi.org/10.1182/blood.V116.21.4385.4385.
Walenga JM, Jeske WP, Hoppensteadt D, Cunanan J, Khan H, Escalante V, et al. Comparative Studies on Branded Enoxaparin and a US Generic Version of Enoxaparin. Clin Appl Thromb 2013;19:261–7. DOI:https://doi.org/10.1177/1076029612463427.
Pavoni V, Gianesello L, Conti D, Ballo P, Dattolo P, Prisco D, et al. “In Less than No Time”: Feasibility of Rotational Thromboelastometry to Detect Anticoagulant Drugs Activity and to Guide Reversal Therapy. J Clin Med 2022;11:1407. DOI:https://doi.org/10.3390/jcm11051407.
Czempik PF, Beberok A. Effect of prophylactic doses of enoxaparin on antifactor Xa activity confirmed by rotational thromboelastometry in critically ill patients: a preliminary prospective cohort study. Front Pharmacol 2025;15:1498188. DOI:https://doi.org/10.3389/fphar.2024.1498188.
Brouwers JRBJ, Roeters Van Lennep JE, Beinema MJ. Biosimilars of low molecular weight heparins: Relevant background information for your drug formulary. Br J Clin Pharmacol 2019;85:2479–86. DOI: https://doi.org/10.1111/bcp.14081.
Kurki P, Van Aerts L, Wolff-Holz E, Giezen T, Skibeli V, Weise M. Interchangeability of Biosimilars: A European Perspective. BioDrugs 2017;31:83–91. DOI:https://doi.org/10.1007/s40259-017-0210-0.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Andrés F. Zuluaga, Ivone Jiménez Toro, Andres Hinc`apie

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Copyright Notice and Open Access Statement
The Journal Vitae works under the Open Access license, and the published manuscripts remain available for the public, both on the Journal's website and in databases, under the Creative Commons license, "Noncommercial Attribution" and "Share alike" systems, adopted in Colombia. Hence, when the authors agree to publish in the Journal Vitae, they will not have the right to economic retributions on publications and reproductions through different diffusion media. The documents are freely available to the internet public, permitting users to read, download, copy, distribute, print, search, or link to the full texts and pass them as data to software. The only constraint on reproduction and distribution, should be to give authors control over the integrity of their work and the right to be appropriately acknowledged and cited.
Authors declare that:
-
They are the intellectual property owners and are responsible for all the information stated in the article.
-
This manuscript has not been submitted or published in other printed or digital media. They accept the responsibility for the judgments, opinions, and points of view expressed in the published article and, therefore, they exonerate Universidad de Antioquia and Journal Vitae from any process.
-
They exempt Universidad de Antioquia and Journal Vitae from settling conflicts or disputes related to the authorship of the referred article.
-
They accept the revision of the original manuscript by suitable personnel, and they bind themselves to perform the corrections appointed or suggested by the assessors.
-
Therefore, they know the editorial process and will not bind the Editorial Board of the Journal to assume any obligations regarding the volume and issue in which the article is published.
-
They transfer the rights of publication, reprinting, and distribution of the article from the moment of its approval, in print and digital format, without the right to economic rewards, and under the licensing conditions considered relevant by Journal Vitae.
-
They fully authorize Universidad de Antioquia and Journal Vitae to submit the published material to the diverse databases and indexing systems where the Journal can be found to comply with the requirements of the regulatory authorities to maintain the national classification of journals.
-
They will assume the article publication costs established for the current issue, and they will make the payment as soon as they are informed about the volume and the issue in which the final version of the article is published.
-
After the article is published, you can share digital or printed copies in a noncommercial manner. You will be able to use the paper in your institution or company for educational or research purposes, including the use in course programs.
Conflict of interest: Authors are responsible for recognizing and disclosing any financial or other benefits that could be perceived to bias their work, acknowledging all financial support and any personal connections with potential sponsors. Examples of such conflicts include receiving research funds or honoraria, serving on advisory boards, stock ownership, or employment and consulting arrangements. Authors without such connections should clearly state that they have no financial support or personal relationships that could be perceived to bias their work. All conflicts of interest should be disclosed on the author's identification page of the manuscript.









