Propolis from native Stingless Bees: ultrasound-assisted extraction
DOI:
https://doi.org/10.17533/udea.vitae.v29n2a347446Keywords:
Propolis extract, Antioxidant activity, Antibacterial activity, Melipona eburnea, Scaptotrigona spp, Tetragonisca angustula, S. aureus, E. coliAbstract
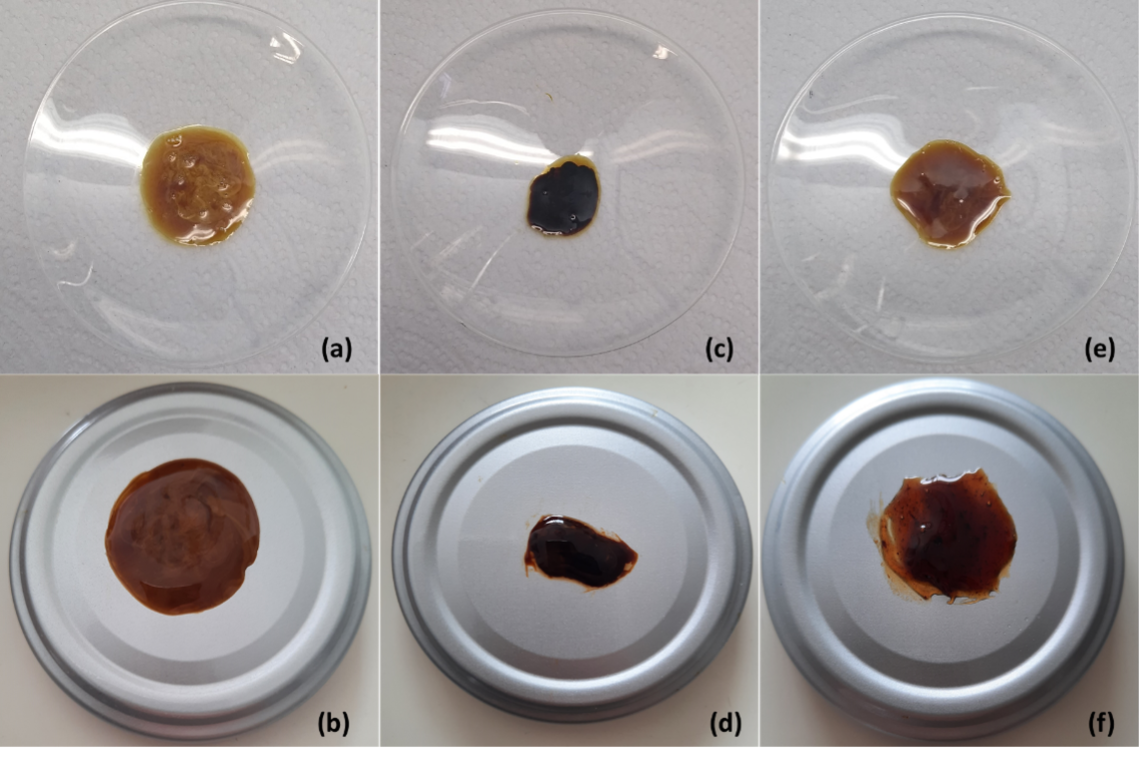
BACKGROUND: Propolis has been considered a highly valuable material due to its therapeutic properties. However, in Colombia, the commercialization of propolis is limited not only by low production but also by the little knowledge about its efficient extraction. Therefore, finding an optimal and economical extraction method to obtain propolis is a necessity for beekeepers that would open new possibilities for industrial use and, therefore, for the market. OBJECTIVES: The objective of this study was to evaluate a conventional and ultrasound-assisted extraction method, seeking to obtain the highest yield and a high amount of content of bioactive compounds in propolis extracts. METHODS: The extraction was carried out for three crude propolis from different types of bees: Tetragonisca angustula or Angelita (ANG), Melipona eburnea, or Melipona (MEL), and Scaptotrigona spp (SCT). The extracts were characterized by color, pH, visual appearance, solid content, antioxidant capacity, total polyphenol content, and bacterial inhibition capacity. RESULTS: The highest extraction performance was obtained when the ultrasound-assisted method was used, especially for the ANG extract, which in addition to presenting inhibition for gram-negative (E. coli) and gram-positive (S. Aureus) bacteria, had the best antioxidant activity with a value of 545 mg GAE / 100 g of sample and total polyphenol content of 1,884 mg GAE / 100 g of sample. CONCLUSIONS: Ultrasound-assisted extraction can be considered a low-cost alternative to increase the extraction performance of crude propolis, together with its total polyphenol content and antioxidant capacity, without altering its physical properties.
Downloads
References
Sun-Waterhouse D. The development of fruit-based functional foods targeting the health and wellness market: a review. Int J Food Sci Technol. 2011;46(5):899–920. DOI: https://doi.org/10.1111/j.1365-2621.2010.02499.x
Bastos E, Guzmán D, Figueroa J, Tello J, Scoaris D. Caracterización antimicrobiana y fisicoquímica de propóleos de Apis mellifera L. (Hymenoptera: Apidae) de la región andina Colombiana. Acta Biológica Colomb. 2011;16(1):175–83
Mora DPP, Santiago KB, Conti BJ, de Oliveira Cardoso E, Conte FL, Oliveira LPG. The chemical composition and events related to the cytotoxic effects of propolis on osteosarcoma cells: A comparative assessment of Colombian samples. Phyther Res. 2019;33(3):591–601. DOI: https://doi.org/10.1002/ptr.6246
Havsteen BH. The biochemistry and medical significance of the flavonoids. Pharmacol Ther. 2002;96(2–3):67–202. DOI: https://doi.org/10.1016/S0163-7258(02)00298-X
Kosalec I, Pepeljnjak S, Bakmaz M, Vladimir-Knežević S. Flavonoid analysis and antimicrobial activity of commercially available propolis products. Acta Pharm. 2005;55(4):423–30
Gonçalves GMS, Srebernich SM, Souza JA de M. Stability and sensory assessment of emulsions containing propolis extract and/or tocopheryl acetate. Brazilian J Pharm Sci. 2011;47(3):585–92. DOI: https://doi.org/10.1590/S1984-82502011000300016
Rogina-Car B, Rogina J, Govorčin Bajsić E, Budimir A. Propolis–Eco-friendly natural antibacterial finish for nonwoven fabrics for medical application. J Ind Text. 2018;49(8): 1100-1119. DOI: https://doi.org/10.1177/1528083718805711
Tosi EA, Ré E, Ortega ME, Cazzoli AF. Food preservative based on propolis: Bacteriostatic activity of propolis polyphenols and flavonoids upon Escherichia coli. Food Chem. 2007;104(3):1025–1029. DOI: https://doi.org/10.1016/j.foodchem.2007.01.011
Sanches MA, Pereira AMS, Serrão JE. Acciones farmacológicas de extractos de propóleos de abejas sin aguijón (Meliponini). J Apic Res. 2017;56(1):50–7. DOI: http://dx.doi.org/10.1080/00218839.2016.1260856
Popova M, Trusheva B, Bankova V. Propolis of stingless bees: A phytochemist’s guide through the jungle of tropical biodiversity. Phytomedicine. 2021;86:153098. DOI: https://doi.org/10.1016/j.phymed.2019.153098
Franchin M, Rosalen PL, Da Cunha MG, Silva RL, Colón DF, Bassi GS. Cinnamoyloxy-mammeisin Isolated from Geopropolis Attenuates Inflammatory Process by Inhibiting Cytokine Production: Involvement of MAPK, AP-1, and NF-κB. J Nat Prod. 2016;79(7):1828–33. DOI: https://doi.org/10.1021/acs.jnatprod.6b00263
Carneiro MJ, López BGC, Lancellotti M, Franchi GC, Nowill AE, Sawaya ACHF. Evaluación de la composición química y la actividad biológica de los extractos de propóleos de Tetragonisca angustula y Schinus terebinthifolius Raddi (Anacardiaceae). J Apic Res. 2016;55(4):315–323. DOI: http://dx.doi.org/10.1080/00218839.2016.1243295
Popova M, Gerginova D, Trusheva B, Simova S, Tamfu AN, Ceylan O. A preliminary study of chemical profiles of honey, cerumen, and propolis of the african stingless bee Meliponula ferruginea. Foods. 2021;10(5). DOI: https://doi.org/10.3390/foods10050997
López-Patiño C. Globalización y producción de propóleos. Biotecnol en el Sect Agropecu. 2011;9(1):119-125. http://www.scielo.org.co/scielo.php?script=sci_arttext&pid=S1692-5612011000100015
Yeo KL, Leo CP, Chan DJC. Ultrasonic enhancement on propolis extraction at varied pH and alcohol content. J Food Process Eng. 2015;38(6):562–570. DOI: https://doi.org/10.1111/jfpe.12186
Dzah CS, Duan Y, Zhang H, Wen C, Zhang J, Chen G. The effects of ultrasound assisted extraction on yield, antioxidant, anticancer and antimicrobial activity of polyphenol extracts: A review. Food Biosci. 2020;35:100547. DOI: https://doi.org/10.1016/j.fbio.2020.100547
Belwal T, Ezzat SM, Rastrelli L, Bhatt ID, Daglia M, Baldi A. A critical analysis of extraction techniques used for botanicals: Trends, priorities, industrial uses and optimization strategies. TrAC Trends Anal Chem. 2018;100:82–102. DOI: https://doi.org/10.1016/j.trac.2017.12.018
Pobiega K, Kraśniewska K, Derewiaka D, Gniewosz M. Comparison of the antimicrobial activity of propolis extracts obtained by means of various extraction methods. J Food Sci Technol. 2019;56(12):5386–5395. DOI: https://doi.org/10.1007/s13197-019-04009-9
Singleton VL, Orthofer R, Lamuela-Raventós RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999;299:152–178. DOI: https://doi.org/10.1016/S0076-6879(99)99017-1
Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci Technol. 1995;28(1):25–30. DOI: https://doi.org/10.1016/S0023-6438(95)80008-5
Pranoto Y, Rakshit SK, Salokhe VM. Enhancing antimicrobial activity of chitosan films by incorporating garlic oil, potassium sorbate and nisin. LWT-Food Sci Technol. 2005;38(8):859–865. DOI: https://doi.org/10.1016/j.lwt.2004.09.014
Choe J-H, Kim H-Y, Kim Y-J, Yeo E-J, Kim C-J. Antioxidant activity and phenolic content of persimmon peel extracted with different levels of ethanol. Int J Food Prop. 2014;17(8):1779–1790. DOI: https://doi.org/10.1080/10942912.2012.731460
Oroian M, Ursachi F, Dranca F. Influence of ultrasonic amplitude, temperature, time and solvent concentration on bioactive compounds extraction from propolis. Ultrason Sonochem. 2020;64:105021. DOI: https://doi.org/10.1016/j.ultsonch.2020.105021
Yuan Y, Zheng S, Zeng L, Deng Z, Zhang B, Li H. The Phenolic Compounds, Metabolites, and Antioxidant Activity of Propolis Extracted by Ultrasound-Assisted Method. J Food Sci. 2019;84(12):3850–3865. DOI: https://doi.org/10.1111/1750-3841.14934
Md-Zin NB, Azemin A, Mohd Rodi MM, Mohd KS. Chemical composition and antioxidant activity of stingless bee propolis from different extraction methods. Int J Eng Technol. 2018;7(4.43):90–95. DOI: https://doi.org/10.14419/ijet.v7i4.43.25825
Rebiai A, Lanez T, Belfar ML. Total polyphenol contents, radical scavenging and cyclic voltammetry of algerian propolis. Int J Pharm Pharm Sci. 2014;6(1):395–400.
Khacha-ananda S, Tragoolpua K, Chantawannakul P, Tragoolpua Y. Antioxidant and anti-cancer cell proliferation activity of propolis extracts from two extraction methods. Asian Pacific J Cancer Prev. 2013;14(11):6991–6995. DOI: https://doi.org/10.7314/apjcp.2013.14.11.6991
Trusheva B, Trunkova D, Bankova V. Different extraction methods of biologically active components from propolis; a preliminary study. Chem Cent J. 2007;1(1):1–4. DOI: https://doi.org/10.1186/1752-153X-1-13
Goltz C, Ávila S, Barbieri JB, Igarashi-Mafra L, Mafra MR. Ultrasound-assisted extraction of phenolic compounds from Macela (Achyrolcine satureioides) extracts. Ind Crops Prod. 2018;115:227–234. DOI: https://doi.org/10.1016/j.indcrop.2018.02.013
Amirullah NA, Zainal Abidin N, Abdullah N, Manickam S. Application of ultrasound towards improving the composition of phenolic compounds and enhancing in vitro bioactivities of Pleurotus pulmonarius (Fr.) Quél extracts. Biocatal Agric Biotechnol. 2021;31:101881. DOI: https://doi.org/10.1016/j.bcab.2020.101881
Jug M, Končić MZ, Kosalec I. Modulation of antioxidant, chelating and antimicrobial activity of poplar chemo-type propolis by extraction procures. LWT - Food Sci Technol. 2014;57(2):530–537. DOI: http://dx.doi.org/10.1016/j.lwt.2014.02.006
Cunha IBS, Sawaya ACHF, Caetano FM, Shimizu MT, Marcucci MC, Drezza FT. Factors that influence the yield and composition of Brazilian propolis extracts. J Braz Chem Soc. 2004;15(6):964–790. DOI: https://doi.org/10.1590/S0103-50532004000600026
Cibanal I, Krepper G, Fernández L, Gallez L. Caracterización fisico-química de propóleos argentinos para su uso como biofungicida agrícola. IV Congr Int Científico y Tecnológico-CONCYT. 2017;1–12, https://digital.cic.gba.gob.ar/handle/11746/6778
Pujirahayu N, Ritonga H, Uslinawaty Z. Properties and flavonoids content in propolis of some extraction method of raw propolis. Int J Pharm Pharm Sci. 2014;6(6):338–340.
Dias LG, Pereira AP, Estevinho LM. Comparative study of different Portuguese samples of propolis: Pollinic, sensorial, physicochemical, microbiological characterization and antibacterial activity. Food Chem Toxicol. 2012;50(12):4246–4253. DOI: https://doi.org/10.1016/j.fct.2012.08.056
Mello BCBS, Hubinger MD. Antioxidant activity and polyphenol contents in Brazilian green propolis extracts prepared with the use of ethanol and water as solvents in different pH values. Int J Food Sci Technol. 2012;47(12):2510–2518. DOI: https://doi.org/10.1111/j.1365-2621.2012.03129.x
Rodríguez Rodríguez LE, Góngora Amores W, Escalona Arias A, Miranda Bazán MB, Batista Suárez S, Bermúdez Cisnero Y. Optimización de la extracción alcohólica para la obtención de soluciones concentradas de propóleos. Rev Colomb Ciencias Químico-Farmacéuticas. 2015;44(1):47–57. DOI: http://dx.doi.org/10.15446/rcciquifa.v44n1.54237
Ali IH, Daoud AS, Shareef AY. Physical properties and chemical analysis of Iraqi propolis. Tikrit J Pure Sci. 2012;17(2):26–31.
Revilla I, Vivar-Quintana AM, González-Martín I, Escuredo O, Seijo C. The potential of near infrared spectroscopy for determining the phenolic, antioxidant, color and bactericide characteristics of raw propolis. Microchem J. 2017;134:211–217. DOI: https://doi.org/10.1016/j.microc.2017.06.006
Hasan AEZ, Mangunwidjaja D, Sunarti TC, Suparno O, Setiyono A. Investigating the antioxidant and anticytotoxic activities of propolis collected from five regions of Indonesia and their abilities to induce apoptosis. Emirates J Food Agric. 2014;26(5):390–398. DOI: https://doi.org/10.9755/ejfa.v26i5.16549
Choi YM, Noh DO, Cho SY, Suh HJ, Kim KM, Kim JM. Antioxidant and antimicrobial activities of propolis from several regions of Korea. LWT - Food Sci Technol. 2006;39(7):756–761. DOI: https://doi.org/10.1016/j.lwt.2005.05.015
Irigoiti Y, Navarro A, Yamul D, Libonatti C, Tabera A, Basualdo M. The use of propolis as a functional food ingredient: A review. Trends Food Sci Technol. 2021;115:297–306. DOI: https://doi.org/10.1016/j.tifs.2021.06.041
Pobiega K, Kraśniewska K, Przybył JL, Bączek K, Żubernik J, Witrowa-Rajchert D. Growth biocontrol of foodborne pathogens and spoilage microorganisms of food by Polish propolis extracts. Molecules. 2019;24(16):2965. DOI: https://doi.org/10.3390/molecules24162965
Dantas Silva RP, Machado BAS, Barreto G de A, Costa SS, Andrade LN, Amaral RG. Antioxidant, antimicrobial, antiparasitic, and cytotoxic properties of various Brazilian propolis extracts. PLoS One. 2017;12(3):e0172585, DOI: https://doi.org/10.1371/journal.pone.0172585
Seibert JB, Bautista-Silva JP, Amparo TR, Petit A, Pervier P, dos Santos Almeida JC. Development of propolis nanoemulsion with antioxidant and antimicrobial activity for use as a potential natural preservative. Food Chem. 2019;287:61–67. DOI: https://doi.org/10.1016/j.foodchem.2019.02.078
Kadariya J, Smith TC, Thapaliya D. Staphylococcus aureus and staphylococcal food-borne disease: an ongoing challenge in public health. Biomed Res Int. 2014;2014:1-9. DOI: https://doi.org/10.1155/2014/827965
Published
How to Cite
Issue
Section
License
Copyright (c) 2022 Carolina Medina-Jaramillo, Loren Milena Carvajal-Díaz, Alex López-Córdoba

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Copyright Notice and Open Access Statement
The Journal Vitae works under the Open Access license, and the published manuscripts remain available for the public, both on the Journal's website and in databases, under the Creative Commons license, "Noncommercial Attribution" and "Share alike" systems, adopted in Colombia. Hence, when the authors agree to publish in the Journal Vitae, they will not have the right to economic retributions on publications and reproductions through different diffusion media. The documents are freely available to the internet public, permitting users to read, download, copy, distribute, print, search, or link to the full texts and pass them as data to software. The only constraint on reproduction and distribution, should be to give authors control over the integrity of their work and the right to be appropriately acknowledged and cited.
Authors declare that:
-
They are the intellectual property owners and are responsible for all the information stated in the article.
-
This manuscript has not been submitted or published in other printed or digital media. They accept the responsibility for the judgments, opinions, and points of view expressed in the published article and, therefore, they exonerate Universidad de Antioquia and Journal Vitae from any process.
-
They exempt Universidad de Antioquia and Journal Vitae from settling conflicts or disputes related to the authorship of the referred article.
-
They accept the revision of the original manuscript by suitable personnel, and they bind themselves to perform the corrections appointed or suggested by the assessors.
-
Therefore, they know the editorial process and will not bind the Editorial Board of the Journal to assume any obligations regarding the volume and issue in which the article is published.
-
They transfer the rights of publication, reprinting, and distribution of the article from the moment of its approval, in print and digital format, without the right to economic rewards, and under the licensing conditions considered relevant by Journal Vitae.
-
They fully authorize Universidad de Antioquia and Journal Vitae to submit the published material to the diverse databases and indexing systems where the Journal can be found to comply with the requirements of the regulatory authorities to maintain the national classification of journals.
-
They will assume the article publication costs established for the current issue, and they will make the payment as soon as they are informed about the volume and the issue in which the final version of the article is published.
-
After the article is published, you can share digital or printed copies in a noncommercial manner. You will be able to use the paper in your institution or company for educational or research purposes, including the use in course programs.
Conflict of interest: Authors are responsible for recognizing and disclosing any financial or other benefits that could be perceived to bias their work, acknowledging all financial support and any personal connections with potential sponsors. Examples of such conflicts include receiving research funds or honoraria, serving on advisory boards, stock ownership, or employment and consulting arrangements. Authors without such connections should clearly state that they have no financial support or personal relationships that could be perceived to bias their work. All conflicts of interest should be disclosed on the author's identification page of the manuscript.









